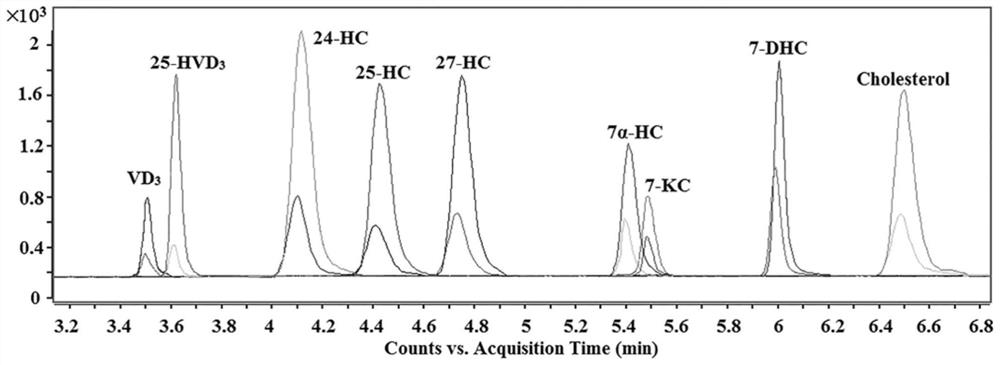
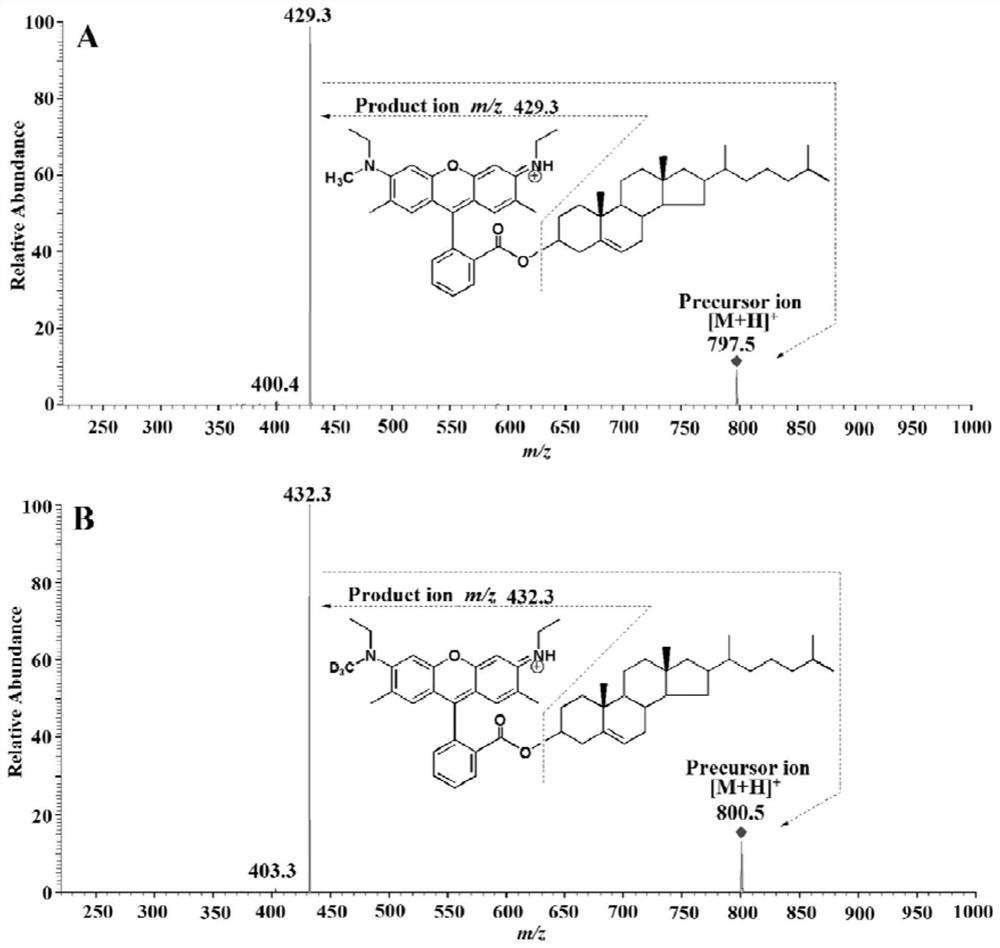
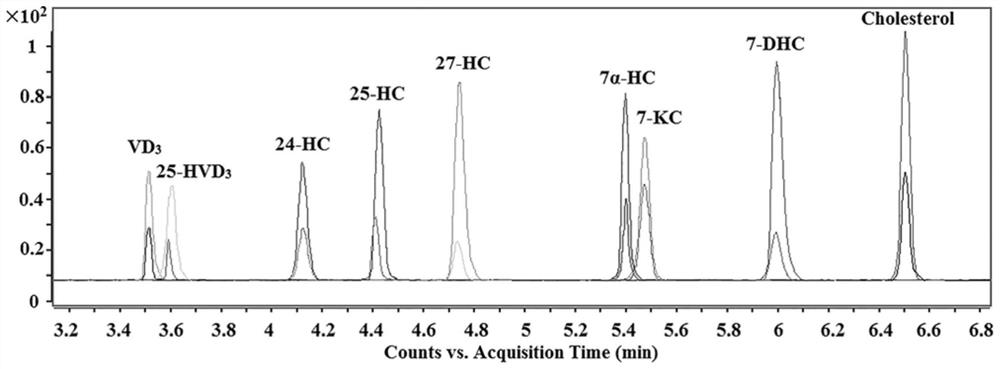
A method for detection and analysis of hydroxyl-containing cholesterol and its metabolites
An analysis method, cholesterol technology, applied in the direction of analyzing materials, measuring devices, material separation, etc., can solve the problems of high price, achieve the effect of improving quantitative accuracy, improving sensitivity, and reducing matrix effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Chromatographic separation and mass spectrometry qualitative and quantitative analysis of hydroxyl-containing cholesterol and its metabolites:
[0039] In the range of 2-3000 pg / mL, prepare 7 different concentrations d 0 - MCR6G-labeled hydroxyl-containing cholesterol and its metabolites standard solution (10 pg / mL, 100 pg / mL, 500 pg / mL, 1000 pg / mL, 1500 pg / mL, 2000 pg / mL, 2500 pg / mL), Among them d 3 -MCR6G-labeled standard (800 pg / mL) was used as fixed internal standard. The specific derivatization process is as follows: 1 part of the above-mentioned mixed standard solution with different concentration levels, 25 μL CMPI (7.5 wt%) and 25 μL DMAP (13 wt%) acetonitrile solution, 100 μL d 0 -MCR6G or d 3 - MCR6G acetonitrile solution, add to a 1.5 mL centrifuge tube one by one, vortex for 10 seconds. Seal and react in a microwave reactor (750 W) at 50 °C for 7.5 min. 7 levels of concentration d 0 -MCR6G mixed standard derivative and d 3 -MCR6G-labeled immobil...
Embodiment 2
[0044] The extraction of hydroxyl-containing cholesterol and metabolites in plasma includes the following steps:
[0045]Aspirate 500 μL of plasma, add 200 μL of 10% trichloroacetic acid to precipitate protein, shake and centrifuge, take supernatant, blow dry with nitrogen at room temperature, and fully dissolve the residue in 200 μL of acetonitrile for stable isotope labeling derivatization. Take 50 μL plasma acetonitrile solution or standard mixed solution, 25 μL CMPI (5.5 wt%) and 25 μL DMAP (12 wt%) acetonitrile solution into a 1.5 mL centrifuge tube. After vortexing for 10 seconds, add 100 μL d 0 -MCR6G or d 3 - MCR6G in acetonitrile. Shake well, seal and react in a microwave reactor (800W) at 55°C for 7.5 minutes. d 0 -MCR6G-labeled cerebrospinal fluid samples and d 3 -MCR6G labeled standard 1:1 ( V / V ) to mix and shake well. 5.5 mg Fe 3 o 4 / GO was dispersed into the above mixed solution, the pH of the solution was adjusted to 7, and shaken for 4 minutes to ...
Embodiment 3
[0047] The extraction of hydroxyl-containing cholesterol and metabolites in cerebrospinal fluid includes the following steps:
[0048] Take 200 μL of cerebrospinal fluid into a centrifuge tube, blow dry with nitrogen gas at room temperature, and fully dissolve the residue with 200 μL of acetonitrile. Take 50 μL of cerebrospinal fluid acetonitrile reconstitution solution or standard mixed solution, 25 μL of CMPI (6 wt%) and 25 μL of DMAP (14 wt%) acetonitrile solution into a 1.5 mL centrifuge tube. After vortexing for 10 seconds, add 100 μL d 0 -MCR6G or d 3 - MCR6G in acetonitrile. Shake well, seal and react in a microwave reactor (750W) at 50 °C for 7.5 minutes. d 0 -MCR6G-labeled cerebrospinal fluid samples and d 3 -MCR6G labeled standard 1:1 ( V / V ) to mix and shake well. 5 mg Fe 3 o 4 / GO was dispersed into the above mixed solution, the pH of the solution was adjusted to 6, and shaken for 4 minutes to reach adsorption equilibrium. Magnetic separation, decantin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com