Oncolytic virus and application
A technology of oncolytic virus and Newcastle disease virus, applied in the biological field, to achieve the effect of promoting virus-specific immune response, enhancing anti-tumor effect, and precisely targeting tumor anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The carrier loaded with oncolytic virus can enhance the oncolytic ability of the carrier and improve the anti-tumor effect of the carrier on the basis of the tumor-targeting ability. The embodiment of the present invention also provides a preparation method of immune effector cells loaded with oncolytic virus, the preparation method comprising the following steps:
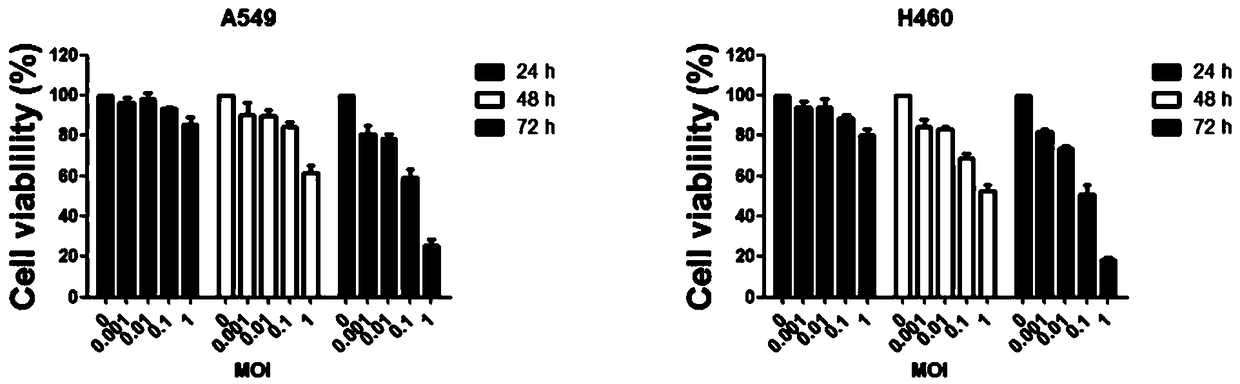
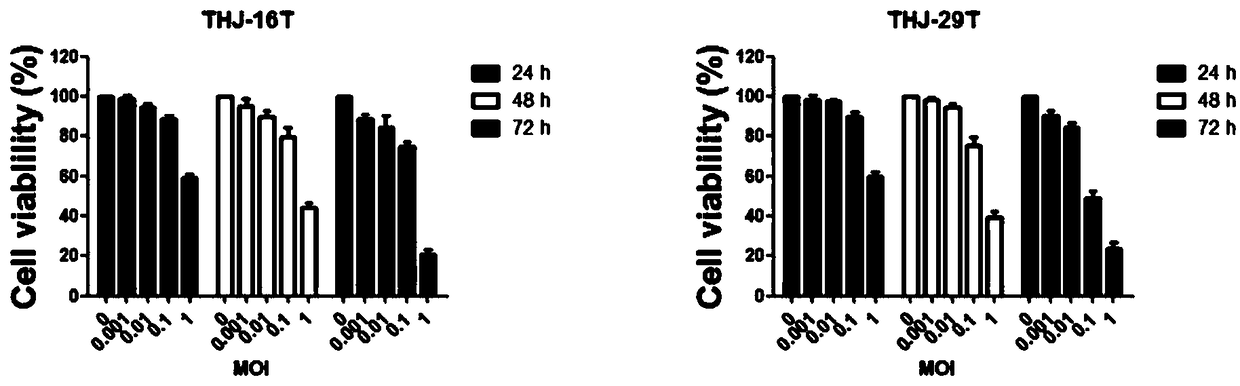
[0039] The preparation method includes contacting an oncolytic virus solution with an immune effector cell solution, and the oncolytic virus in the oncolytic virus solution is the oncolytic virus NDV / FMW provided in the above-mentioned embodiments of the present invention.
[0040] Further, the multiplicity of infection of the oncolytic virus is 10-100 MOI, and the cell density of the immune effector cell solution is 1ⅹ10 6 ~5ⅹ10 6 cells / mL.
[0041] The multiplicity of infection of the oncolytic virus can be: 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38, 40, 42, 44, 46, 48, 50 , 52, 54, 56,...
no. 1 example
[0048] This example provides a method for preparing immune effector cells (CIK cells) loaded with oncolytic virus.
[0049] Induction and proliferation of CIK cells
[0050] Experimental consumables
[0051] X-VIVO 15 serum-free medium: 1000mL / bottle, produced by Lonza, Walkersville, MD, USA; IL-2 (recombinant human interleukin-2): 200,000 IU / bottle and 1 million IU / bottle, produced The business is Shandong Quangang Pharmaceutical Co., Ltd.; IL-1α (recombinant human interleukin-1α): 10 μg / cartridge, the manufacturer is PEPROTECH; CD3 antibody (anti-CD3 monoclonal antibody): 500 μg / cartridge, the manufacturer is e- Bioscience; IFN-γ (recombinant human IFN-γ): 1.0mg / bottle, the manufacturer is PEPROTECH.
[0052] experimental method
[0053] First, experiment preparation, ultraviolet disinfection for 30 minutes. Turn on the exhaust and lighting switches of the ultra-clean workbench, and wipe the workbench with 75% alcohol.
[0054] Collect blood for routine examination of p...
no. 2 example
[0066] Verify that the immune effector cells prepared by the preparation method of the first embodiment are loaded with oncolytic virus.
[0067] experimental method
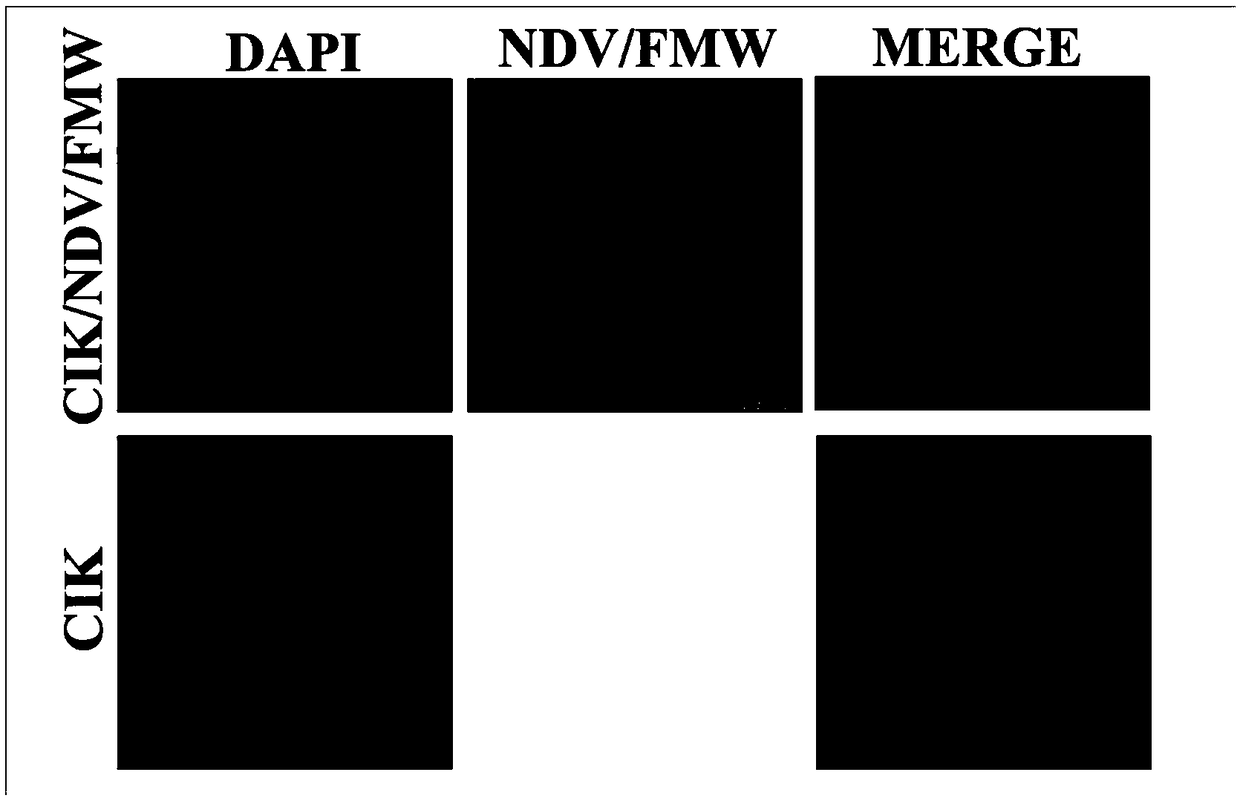
[0068] The immune effector cells CIK cells loaded with oncolytic virus provided in the first embodiment were observed with a laser confocal microscope. Specifically, put cell slides in a six-well plate, inoculate CIK cell suspension (2mL / well), put it in an incubator for pre-culture for 24 hours, and infect CIK cells with 10MOI oncolytic virus (NDV / FMW) at 37°C 4 hours.
[0069] Take out slides, wash twice with PBS, 5 minutes each time; fix with 4% paraformaldehyde for 15 minutes, wash twice with PBS, 5 minutes each time; permeabilize with 0.03% Triton 100 for 10 minutes, wash twice with PBS, 5 minutes each time Minutes; HN (NDV virus protein) antibody incubated overnight at 4°C, washed twice with PBS, 5 minutes each; goat anti-rabbit red fluorescent secondary antibody incubated at room temperature for 30 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com