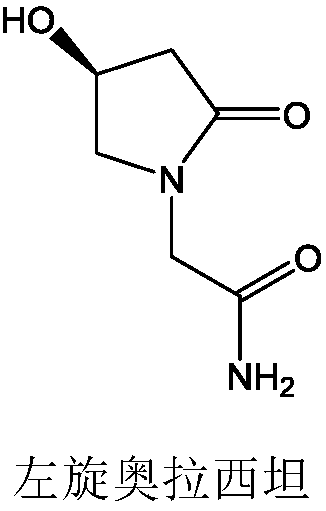
Preparation method of levorotatory oxiracetam orally disintegrating preparation
The technology of oral disintegration and disintegrating agent is applied in the field of preparation of oral disintegrating preparations of levooxiracetam, which can solve the problems of reducing the amount of absorbed drug, increasing the risk of drug safety, unstable pharmacological effect, etc. Small amount, small difference in tablet weight, ensuring the effect of drug efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Get 20 parts of absolute ethanol, under stirring state, add 1 part of levoxiracetam chemical bulk drug (provided by Chongqing Dongze Pharmaceutical Technology Co., Ltd., purity 99.5%) and 6 parts of Eudragit E100 (Guangzhou Shuoheng Biotechnology Co., Ltd. Provided by the company), stirred until dissolved, and then spray-dried in a spray dryer (produced by Huawei Technology Co., Ltd.), the inlet air temperature of spray drying was 138°C, and the outlet air temperature was 79°C; Tan solid dispersion is pulverized to obtain.
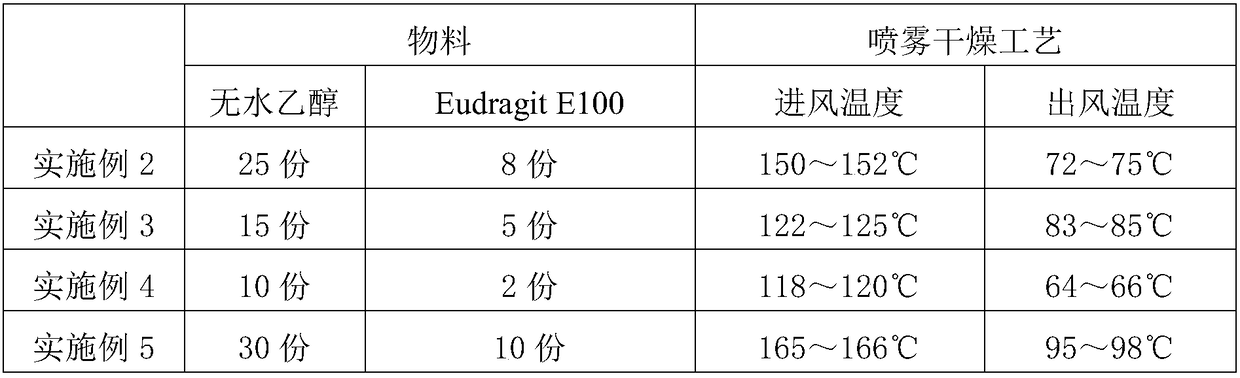
[0033] Referring to the preparation method of Example 1, run Examples 2-5 according to the parameters in Table 1 below to prepare the levoxiracetam solid dispersion. The chemical bulk drug of levoxiracetam used is 1 part.
[0034] Table 1 Preparation of Levoxiracetam Solid Dispersion
[0035]
Embodiment 6
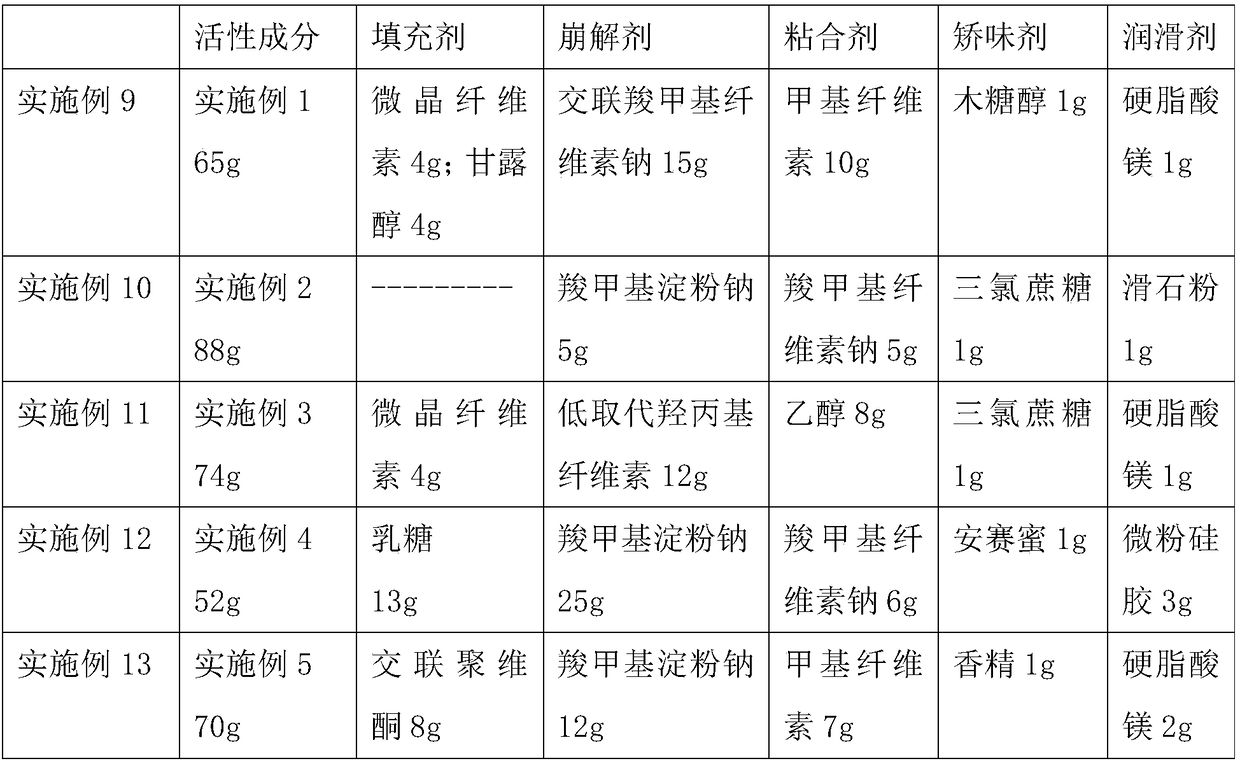
[0037] Prescription: Levoxiracetam solid dispersion prepared in Example 1 70g, pregelatinized starch 4g, mannitol 4g, croscarmellose sodium 4g, low-substituted hydroxypropyl cellulose 8g, polyvinylpyrrolidone 7g , xylitol 1g, micronized silica gel 2g.
[0038] Preparation:
[0039] (1) Material screening: levoxiracetam solid dispersion, pregelatinized starch, mannitol, croscarmellose sodium, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone, xylitol, Micropowder silica gel is passed through a 100-mesh sieve respectively, and set aside;
[0040] (2) Mixing and granulation: Add the sieved levoxiracetam solid dispersion, filler, and disintegrant into a wet granulator (Changzhou Yiyiyi Drying Equipment Co., Ltd.) and mix evenly, start the stirring paddle to mix 5 minutes; stop, add adhesive (polyvinylpyrrolidone ethanol solution prepared with an appropriate amount of ethanol) at one time, start the stirring paddle, mix the granulation paddle to prepare soft material ...
Embodiment 7
[0043] Prescription: Levoxiracetam solid dispersion prepared in Example 1 58g, pregelatinized starch 4g, mannitol 12g, croscarmellose sodium 3g, low-substituted hydroxypropyl cellulose 15g, polyvinylpyrrolidone 6g , sucralose 1g, magnesium stearate 1g.
[0044] Preparation:
[0045] (1) Material screening: solid dispersion of levoxiracetam, pregelatinized starch, mannitol, croscarmellose sodium, low-substituted hydroxypropyl cellulose, polyvinylpyrrolidone, sucralose, Magnesium stearate is passed through a 100-mesh sieve respectively, for subsequent use;
[0046] (2) Mixing and granulation: Add the sieved levoxiracetam solid dispersion and disintegrant into the wet granulator and mix evenly, start the stirring paddle to mix for 10 minutes; stop the machine, and add the binder at one time (polyvinylpyrrolidone ethanol solution prepared with an appropriate amount of ethanol), start the stirring paddle, mix the granulating paddle to prepare soft material for 10 minutes, and dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com