A carbon-doped double metal oxide material and its preparation method
A bimetallic oxide and high-temperature carbonization technology, which is applied in the field of nanomaterials, can solve problems such as poor cycle stability of transition metal oxides, small ion or electron diffusion coefficients, and reduced reversibility of electrode reactions, so as to enhance charge and discharge performance, Low cost, enhanced conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
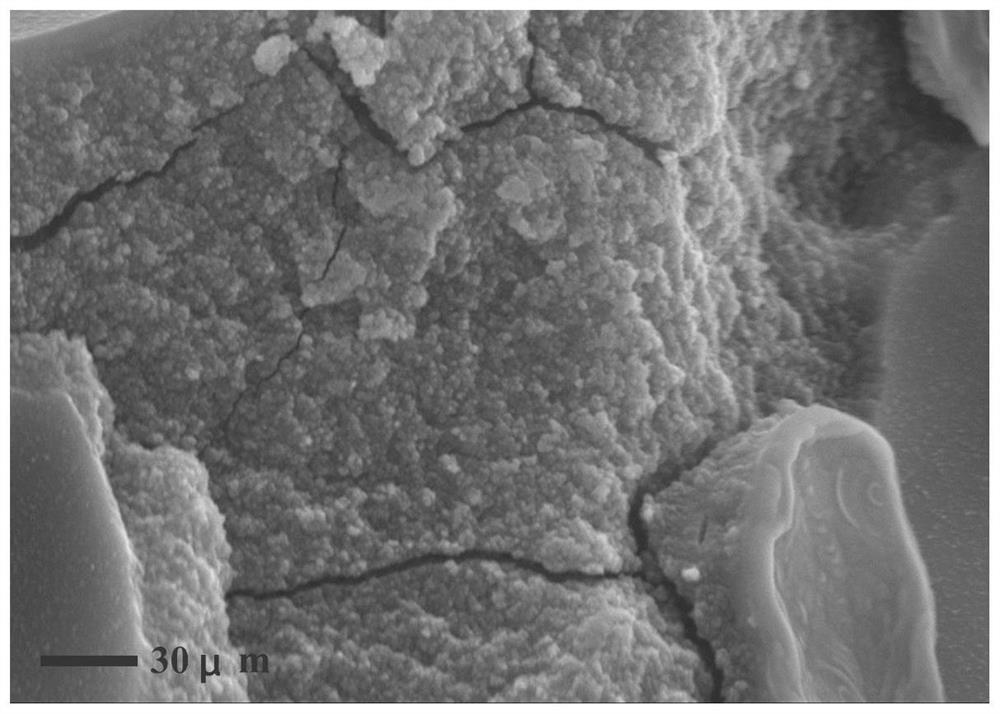
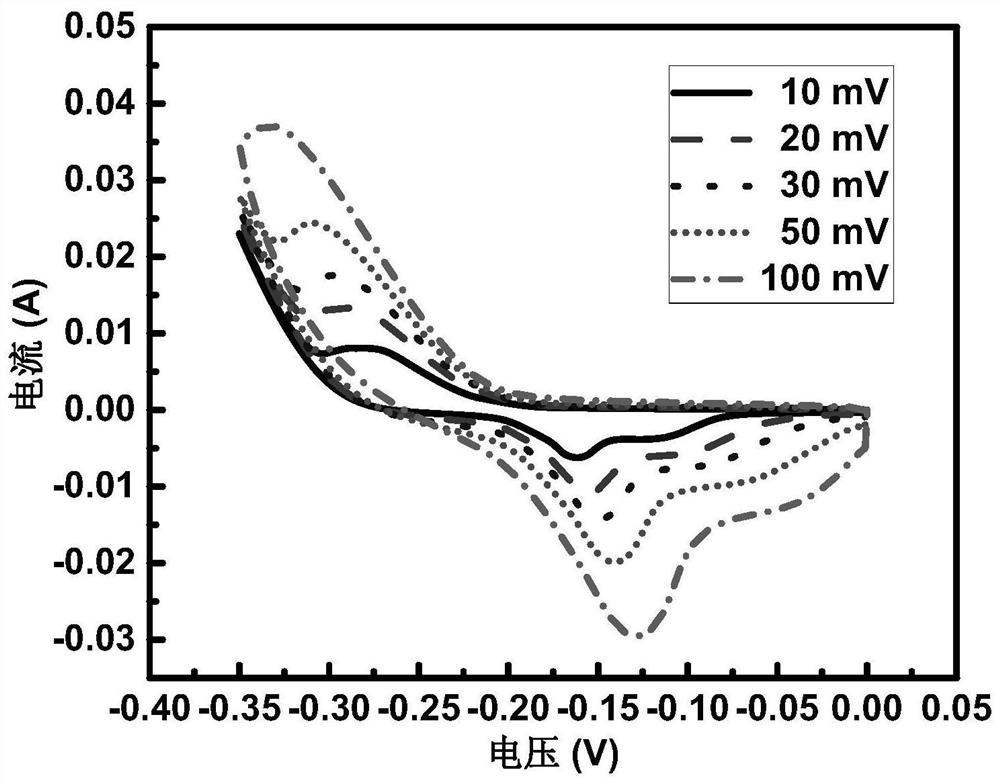
Image
Examples
Embodiment 1
[0025] The raw material formula of carbon-doped double metal oxide material is as follows:
[0026]
[0027] Above-mentioned composite material is prepared by the preparation method of following steps:
[0028] A method for preparing a carbon-doped double metal oxide material, the method comprising the following steps: first heating ferric chloride hexahydrate, nickel chloride hexahydrate, ammonium chloride, gelatin and deionized water to 80°C, fully dissolving Stir evenly, then pour it into a watch glass, and dry it in vacuum at 60°C; then put the dry gel into a quartz boat, place the quartz boat in a tube furnace and heat it under a nitrogen atmosphere with a nitrogen flow rate of 50ml / min. The rate is 5°C / min, slowly heating from room temperature to 300°C, and the carbonization time is 2h; then the hydrochloric acid is prepared into 10ml of 1M solution, etched for 3h, and the carbonized material is cleaned, vacuum-dried at 60°C after suction filtration. Then, the sample...
Embodiment 2
[0032] The raw material formula of carbon-doped double metal oxide material is as follows:
[0033]
[0034]
[0035] Above-mentioned composite material is prepared by the preparation method of following steps:
[0036] A method for preparing a carbon-doped double metal oxide material, the method comprising the following steps: first heating ferric chloride hexahydrate, nickel chloride hexahydrate, ammonium chloride, gelatin, etc. and 10ml deionized water to 85°C, Fully dissolve and stir evenly, then pour into a watch glass, and dry under vacuum at 60°C; then put the dry gel into a quartz boat, place the quartz boat in a tube furnace and heat it under a nitrogen atmosphere with a nitrogen flow rate of 50ml / min , the heating rate was 5°C / min, slowly heated from room temperature to 400°C, and the carbonization time was 2h; then the hydrochloric acid was prepared into a 1M solution, 10ml was etched for 3h, and the carbonized material was cleaned, vacuum-dried at 60°C after ...
Embodiment 3
[0040] The raw material formula of carbon-doped double metal oxide material is as follows:
[0041]
[0042] Above-mentioned composite material is prepared by following steps preparation method:
[0043]A method for preparing a carbon-doped double metal oxide material, the method comprising the following steps: first heating ferric chloride hexahydrate, nickel chloride hexahydrate, ammonium chloride, gelatin and 10ml deionized water to 80°C, Fully dissolve and stir evenly, then pour into a watch glass, and dry under vacuum at 60°C; then put the dry gel into a quartz boat, place the quartz boat in a tube furnace and heat it under a nitrogen atmosphere with a nitrogen flow rate of 50ml / min , the heating rate was 5°C / min, slowly heated from room temperature to 350°C, and the carbonization time was 2h; then the hydrochloric acid was prepared into a 1M solution, 10ml was etched for 3h, and the carbonized material was cleaned, vacuum-dried at 60°C after suction filtration. Then,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com