A method for expressing and purifying recombinant cxcl9 protein and its application
A technology for expression, purification, and protein, which is applied in the field of soluble expression of recombinant CXCL9 protein by the comprehensive use of solubilizing tags and inteins. cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Construction of pET30a / Zbasic-ΔI-CM-CXCL9 plasmid and soluble expression of mCXCL9
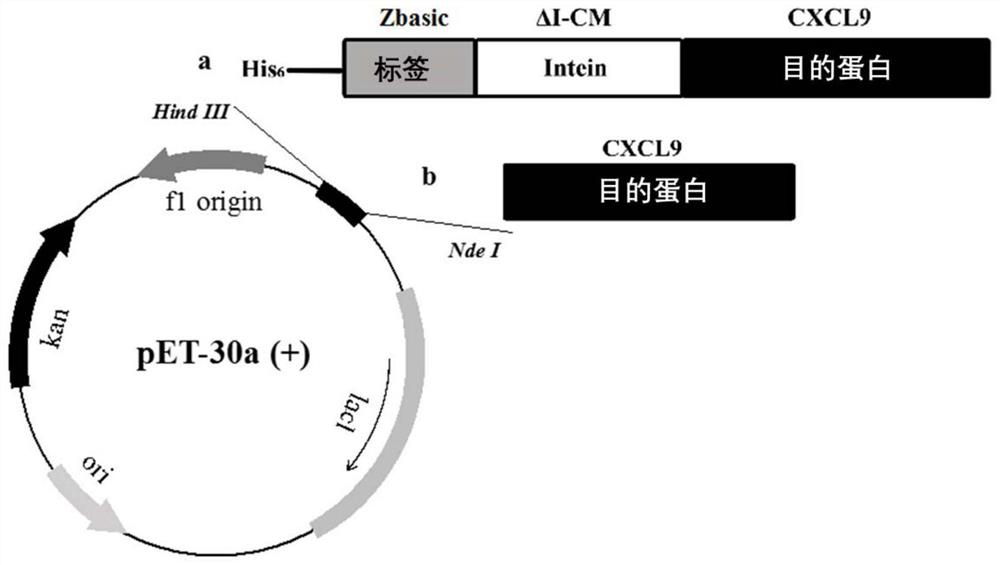
[0051] In this example, we use Zbasic as a solubilizing tag to construct an Intein (ΔI-CM)-mediated fusion protein expression plasmid, and use PCR and enzyme-cut ligation methods to simultaneously ligate and insert the sequences of Zbasic, ΔI-CM, and mCXCL9 in sequence. In the pET30a plasmid, a flexible peptide chain is used between the tag coding sequence and the intein coding sequence, and a separate expression plasmid of PET30a / mCXCL9 is constructed as a control. The construction method is as follows: figure 2 shown.
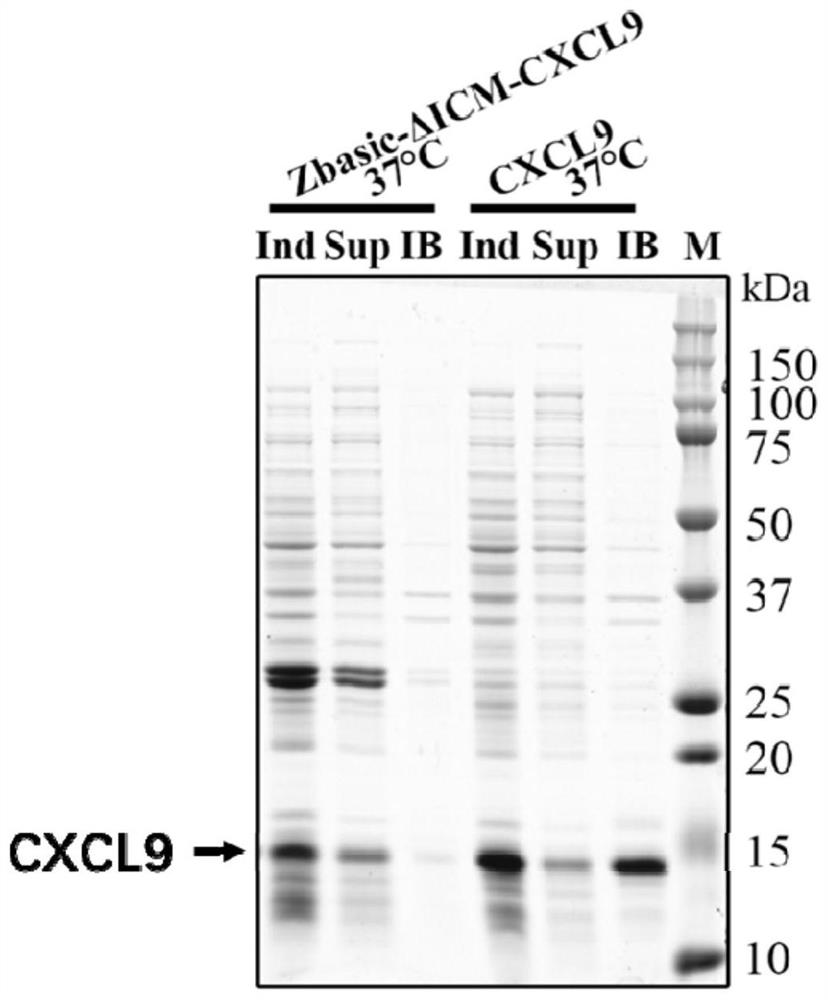
[0052] After the successful construction of the plasmid, transform the BL21(DE3) host bacterium, pick a single clone colony and inoculate it in the LB liquid culture of Kana+, and cultivate it at 200 rpm at 37°C for 12 hours as the seed bacterium. The seed bacteria were inoculated in sterile Kana+LB medium at a volume ratio of 1:100, cultured at 37°C and 200 rpm for ...
Embodiment 2
[0056] Soluble expression of mCXCL9 achieved with additional lytic tags
[0057] In this example, we replaced Zbasic with two other solubilizing tags to verify the extension of the method of the present invention. We selected two tags, FATT (SEQ ID NO.5) and Fh8 (SEQ ID NO.6), and used PCR and enzyme-cut ligation methods to sequentially connect the sequences of the lytic tag, ΔI-CM, and mCXCL9 and insert them into the pET30a vector , obtain pET30a / FATT-ΔI-CM-CXCL9 and pET30a / Fh8-ΔI-CM-CXCL9 plasmids, and transform BL21(DE3) host bacteria, induce expression and detect by SDS-PAGE and Western blot as in the steps of Example 1, Such as Figure 4 shown.
[0058] The detection conditions were as follows: FATT-ΔI-CM-CXCL9 and Fh8-ΔI-CM-CXCL9 were induced to express at 37°C, and the soluble supernatant and inclusion body components were separated for SDS-PAGE (A) and Western blot (B). Lane M: Marker; Lane Ind: Bacteria solution after induction; Lane Sup: Supernatant after lysis; L...
Embodiment 3
[0061] mCXCL9 purification after soluble expression
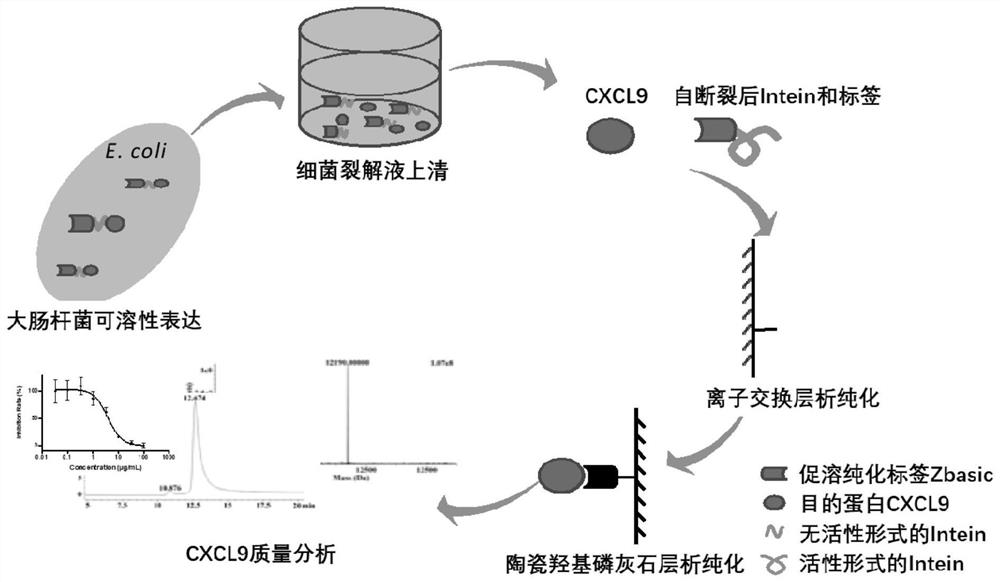
[0062] After the soluble expression of the mCXCL9 protein is obtained by the method of the present invention, it is suitable to be purified by cation exchange chromatography because of its high isoelectric point (PI=10.62). The specific method is as follows: host bacteria expressing m recombinant CXCL9 protein are resuspended to 10% concentration with 50mM PB, pH 7.0 solution, homogeneously broken by high pressure, and centrifuged to obtain supernatant containing soluble mCXCL9. Select SPFF medium for cation exchange purification. First, use SPFF Loading Buffer (50mM PB, 1mM EDTA, 100μM PMSF, pH 7.0) to equilibrate the chromatography column at a flow rate of 1mL / min. After the UV baseline is stable, continue to load the sample at 1mL / min. The protein of interest is bound to the SP FF medium. After loading the sample, continue to wash the column with Loading Buffer, and adjust the solvent to pH 9.0 for elution. Finally, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com