Synthetic method of ketone compounds
The technology of a ketone compound and a synthesis method, which is applied in the field of synthesis of ketone compounds, can solve the problems of difficult synthesis of active intermediates, expensive catalysts, cumbersome synthesis steps, etc., and achieve high synthesis efficiency, simple preparation method, and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
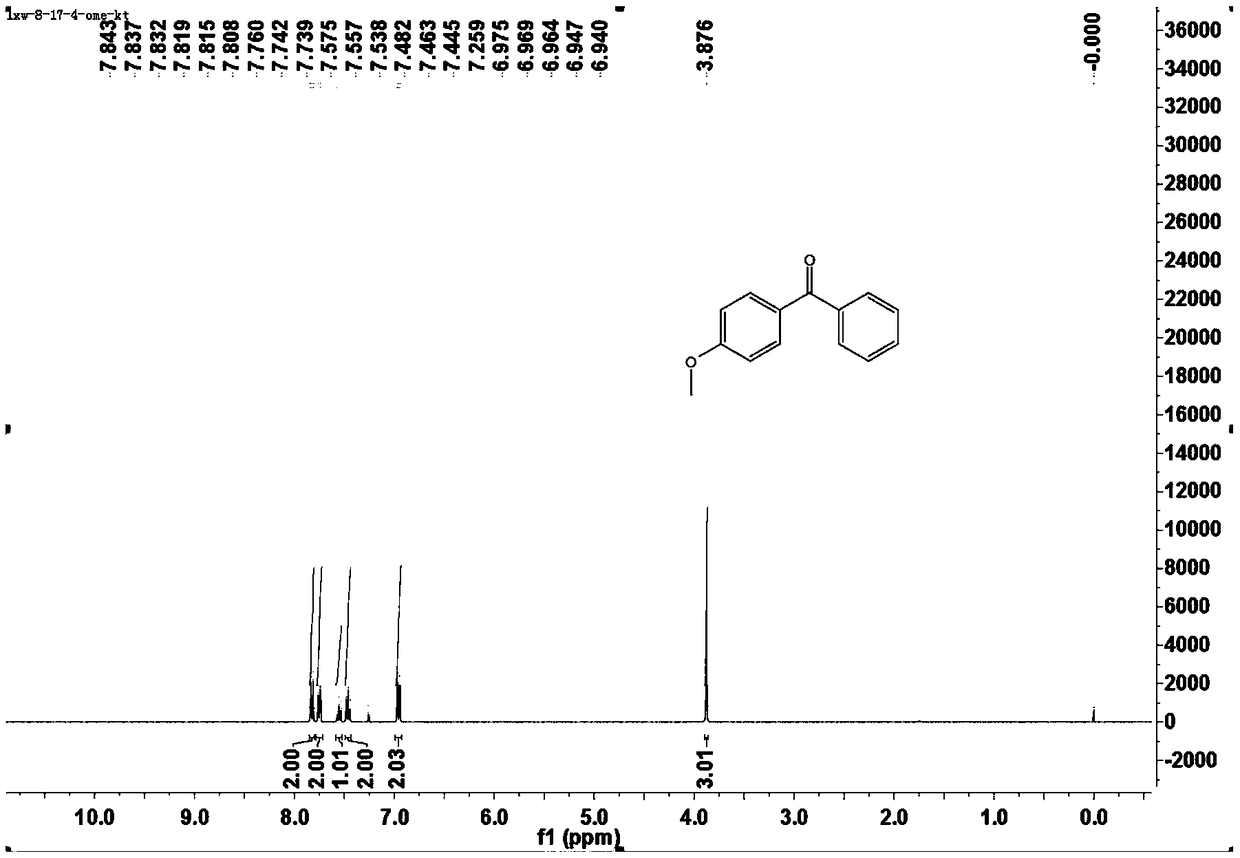
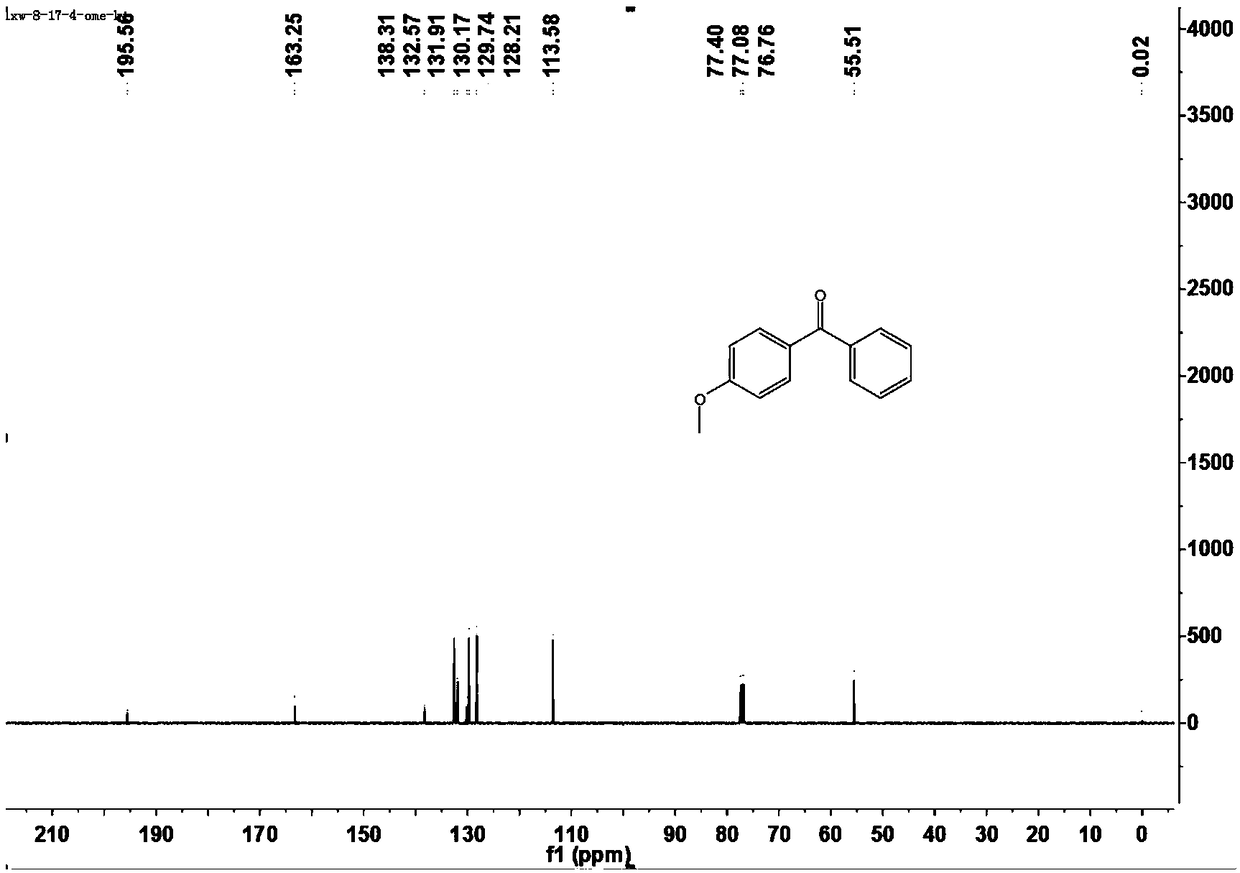
[0043] This example is the specific preparation method and structural analysis of 4-methoxybenzophenone (3aa).
[0044]
[0045]Under an atmosphere of oxygen or air at atmospheric pressure, p-methoxyphenylhydrazine compound 1a (27.6mg, 0.20mmol), carboformic acid 2a (30mg, 0.20mmol), silver acetate (3.5mg, 0.02 mmol), lithium carbonate (15mg, 0.20mmol), 1,2-dichloroethane (DCE, 1mL), react at a temperature of 110°C for 12 hours.
[0046] After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on the prepared silica gel plate, and the selected developer or eluent was petroleum ether and ethyl acetate at a volume ratio of 100:1 to obtain the product 4-methoxybenzophenone 3aa. The yield was 82% (34.8 mg).
[0047] 1 H-NMR (400MHz, CDCl 3 )δ7.84-7.81(m,2H),7.76-7.74(m,1H),7.56(t,J=3.2Hz,1H),7.46(t,J=7.6Hz,2H),6.97-6.94(m ,2H), 3.88...
Embodiment 2
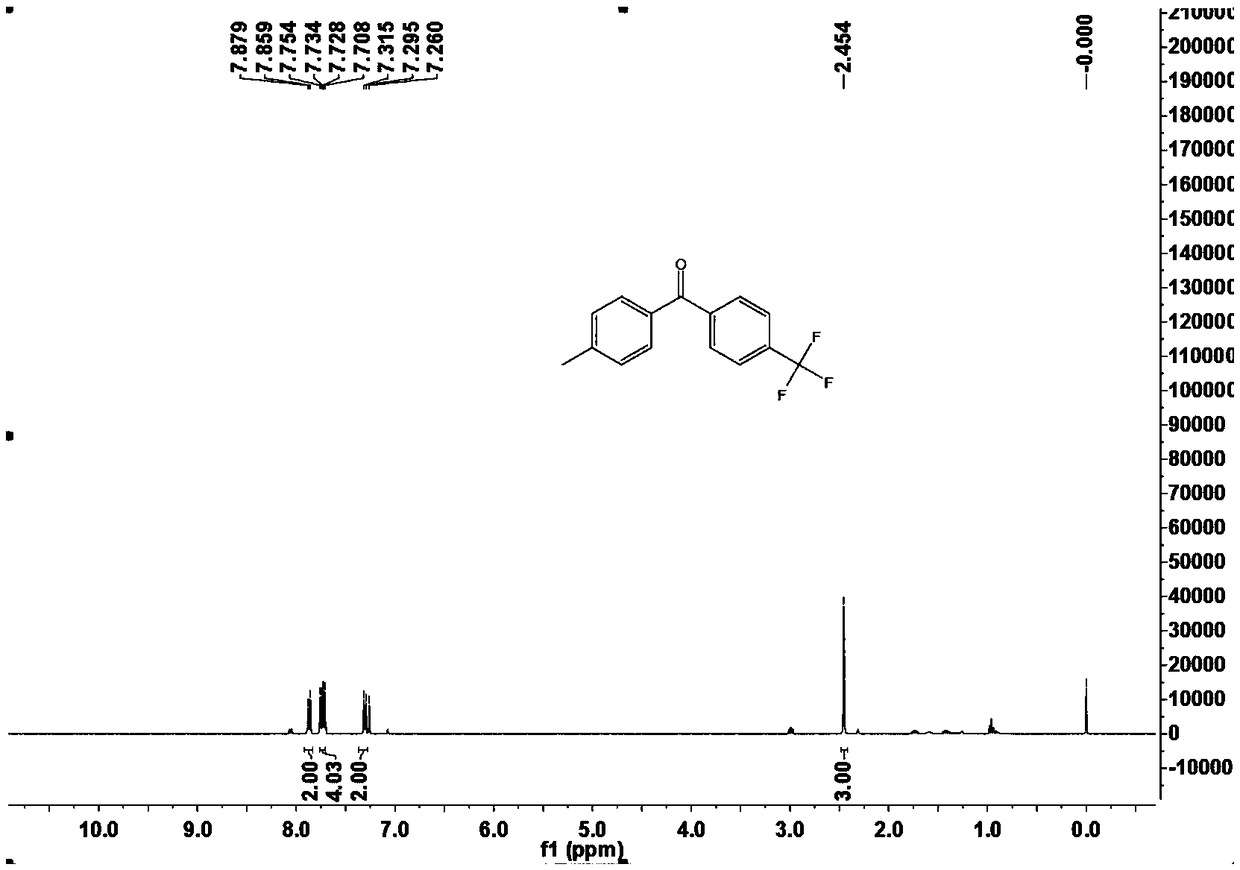
[0050] This example is the specific preparation method and structural analysis of 4-methyl-4'-trifluoromethyl-benzophenone (3bb).
[0051]
[0052] Under an atmosphere of oxygen at atmospheric pressure, p-methoxyphenylhydrazine compound 1b (24.4mg, 0.20mmol), carboformic acid 2b (64.8mg, 0.30mmol), silver acetate (3.5mg, 0.02mmol) were successively added to a 15mL Schlenk reaction tube ), lithium carbonate (15mg, 0.20mmol), 1,2-dichloroethane (DCE, 1mL), react at a temperature of 110°C for 12 hours.
[0053] After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on the prepared silica gel plate, and the selected developer or eluent was petroleum ether and ethyl acetate at a volume ratio of 50:1 to obtain the product 4-methyl-4'-trifluoromethyl- Benzophenone 3bb, yield 75% (39.6mg).
[0054] 1 H-NMR (400MHz, CDCl 3 ) δ 7.88 (d, ...
Embodiment 3
[0057] This example is the specific preparation method and structural analysis of 4-chloro-4'-tert-butyl-benzophenone (3cc).
[0058]
[0059] Under an atmosphere of oxygen or air at atmospheric pressure, p-methoxyphenylhydrazine compound 1c (28.4mg, 0.20mmol), carboformic acid 2c (60.4mg, 0.30mmol), silver acetate (3.5mg, 0.02mmol), lithium carbonate (15mg, 0.20mmol), 1,2-dichloroethane (DCE, 1mL), react at a temperature of 110°C for 12 hours.
[0060] After the reaction was completed, it was cooled to room temperature, filtered through diatomaceous earth, and concentrated to obtain a crude product. The crude product was separated by chromatography on the prepared silica gel plate, and the selected developing solvent or eluent was petroleum ether and ethyl acetate at a volume ratio of 100:1 to obtain the product 4-chloro-4'-tert-butyl-diphenyl Methanone 3cc, yield 76% (41.3mg).
[0061] 1 H-NMR (400MHz, CDCl 3 ) δ7.77-7.72 (m, 4H), 7.51-7.49 (m, 2H), 7.46-7.44 (m, 2H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com