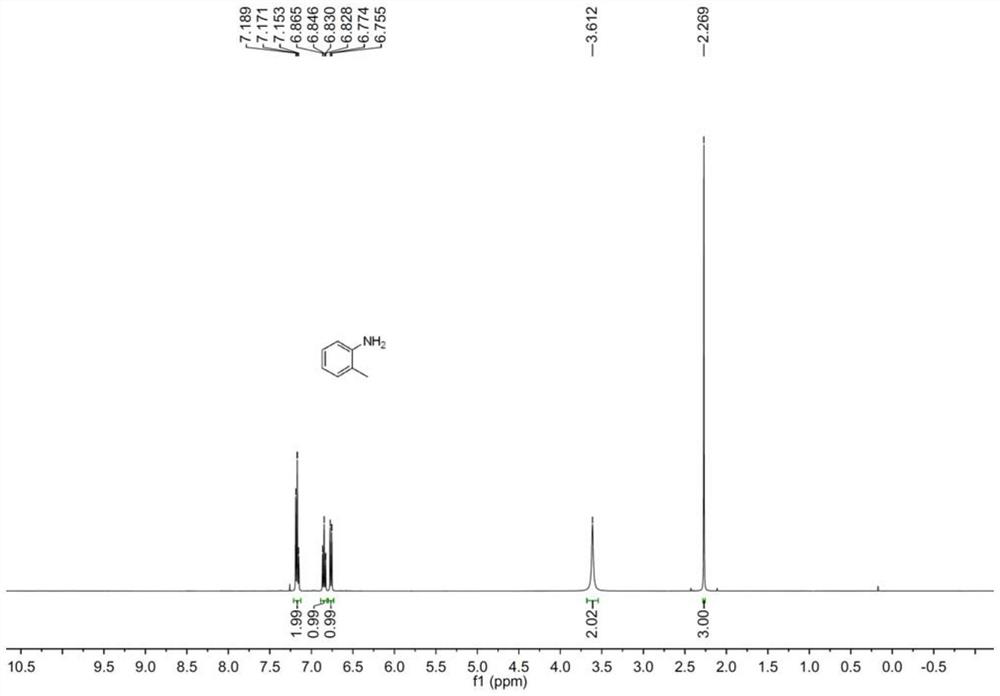
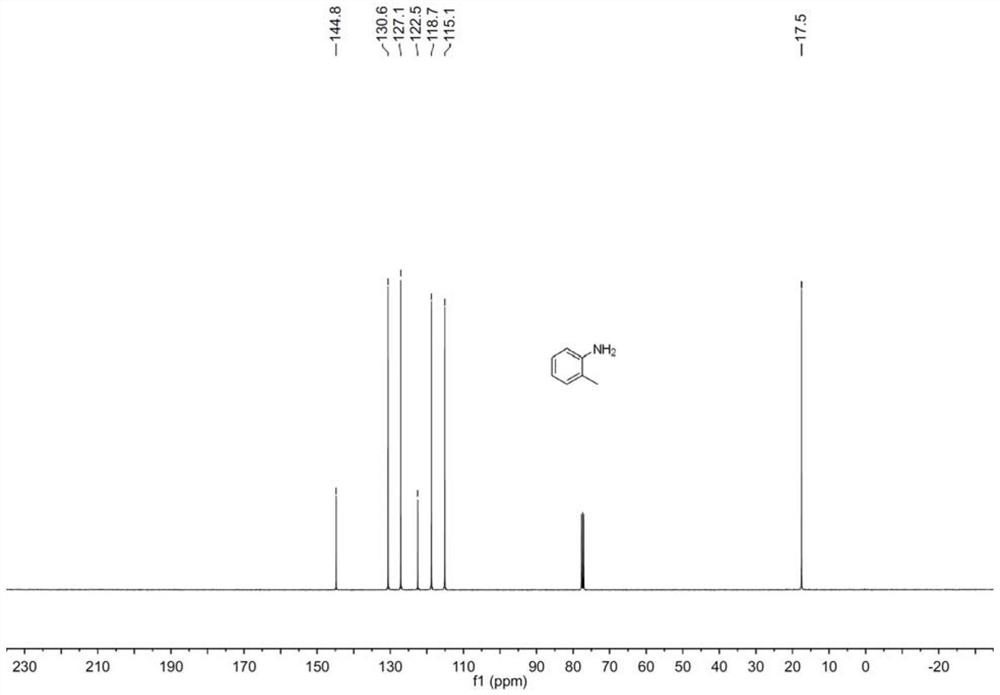
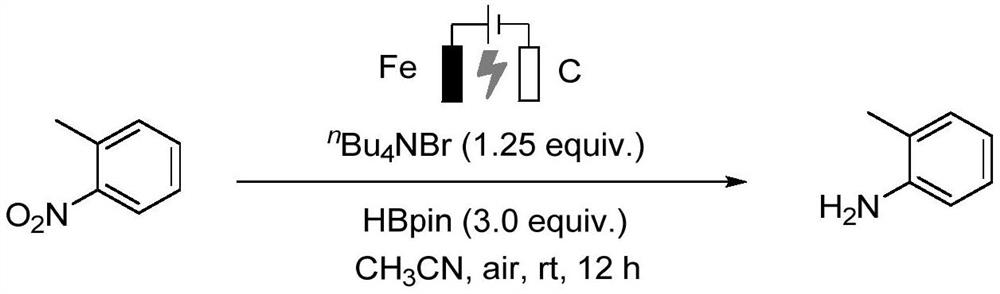
Preparation method of aniline derivative
A technology for aniline derivatives and nitro compounds, applied in the field of preparation of aniline derivatives, can solve the problems of high cost, environmental pollution, high energy consumption and the like, and achieve the effects of high value, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0016] Specific embodiment one: the preparation method of a kind of aniline derivative of this embodiment, concretely carry out according to the following steps:
[0017] 1. Mix the nitro compound, boron reagent, electrolyte and organic solvent evenly to obtain a mixed solution;
[0018] 2. Put two electrodes into the mixed solution obtained in step 1, turn on the power supply, and carry out electrocatalytic reaction with stirring under room temperature and air atmosphere conditions to obtain a reaction mixture;
[0019] 3. The reaction mixture obtained in step 2 is subjected to vacuum distillation to remove the solvent, and is added with silica gel and stirred evenly to obtain a crude product;
[0020] 4. Purify the crude product obtained in step 3 by silica gel column chromatography to obtain aniline derivatives and complete the preparation.
specific Embodiment approach 2
[0021] Specific embodiment two: the difference between this embodiment and specific embodiment one is: the structural formula of the nitro compound described in step one is: wherein R is aryl, heteroaryl or nitrobenzene. Others are the same as in the first embodiment.
specific Embodiment approach 3
[0022] Embodiment 3: This embodiment differs from Embodiment 1 or Embodiment 2 in that: the boron reagent in Step 1 is 3,3,4,4-tetramethylborane. Others are the same as in the first or second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com