A kind of preparation method of medical cell repairing agent
A repairing agent and cell technology, applied in the field of preparation of medical cell repairing agents, can solve the problems of allergies, non-skin substitutes, hidden safety hazards of animal origin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of human amniotic mesenchymal stem cells
[0036] Take aseptically collected amniotic membrane tissue, and wash it in pre-cooled PBS in a biological safety cabinet. If the placenta is accompanied by chorion, the chorion and amnion need to be separated first, the amnion is taken out, and the chorion is discarded; Add an equal volume of 2U / ml neutral protease to digest for 30min, then add a 1:1 mixture of 5.5mg / ml V-type collagenase and 2U / ml hyaluronidase twice and a half volume of amniotic membrane to digest for 3.5h; the collection and digestion are complete The cell suspension was added to the balance solution without calcium and magnesium ions, washed several times, counted the mononuclear cells after dilution with glacial acetic acid, and obtained the total number of cells; adjusted the cell concentration to 1.0×10 5 cells / ml, add serum-free mesenchymal stem cell medium, place at 37°C, the concentration is 5% CO 2, cultured in an incubator with satur...
Embodiment 2
[0043] 1. Preparation of human amniotic mesenchymal stem cells
[0044] Take aseptically collected amniotic membrane tissue, and wash it in pre-cooled PBS in a biological safety cabinet. If the placenta is accompanied by chorion, the chorion and amnion need to be separated first, the amnion is taken out, and the chorion is discarded; Add an equal volume of 2U / ml neutral protease to digest for 30min, then add a 1:1 mixture of 5.5mg / ml V-type collagenase and 2U / ml hyaluronidase twice and a half volume of amniotic membrane to digest for 3.5h; the collection and digestion are complete The cell suspension was added to the balance solution without calcium and magnesium ions, washed several times, counted the mononuclear cells after dilution with glacial acetic acid, and obtained the total number of cells; adjusted the cell concentration to 1.0×10 5 cells / ml, add serum-free mesenchymal stem cell medium, place at 37°C, the concentration is 5% CO 2 , cultured in an incubator with satu...
Embodiment 3
[0050] Embodiment 3 application effect detection
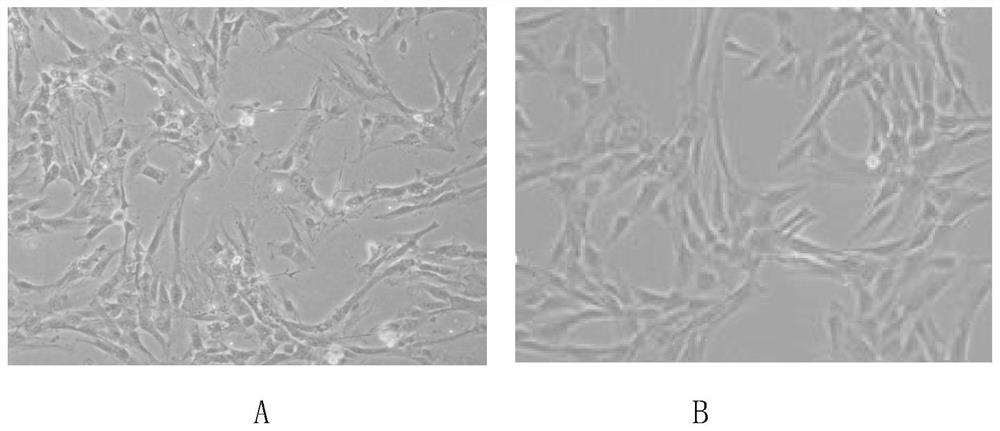
[0051] 1. Biological characteristics of human amniotic mesenchymal stem cells prepared in Example 1:
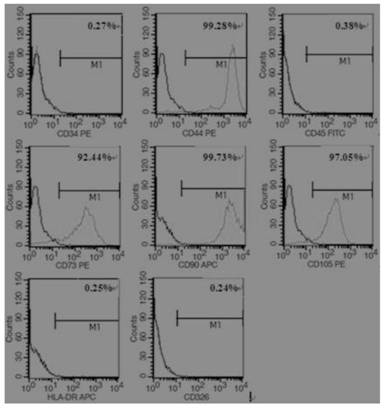
[0052] a. Identification of molecular surface markers by flow cytometry:
[0053] The third-generation amniotic mesenchymal stem cells in good growth state were taken for flow cytometric analysis to see whether they expressed CD34, CD44, CD45, CD73, CD90, CD105, CD326, HLA-DR and other surface molecules.
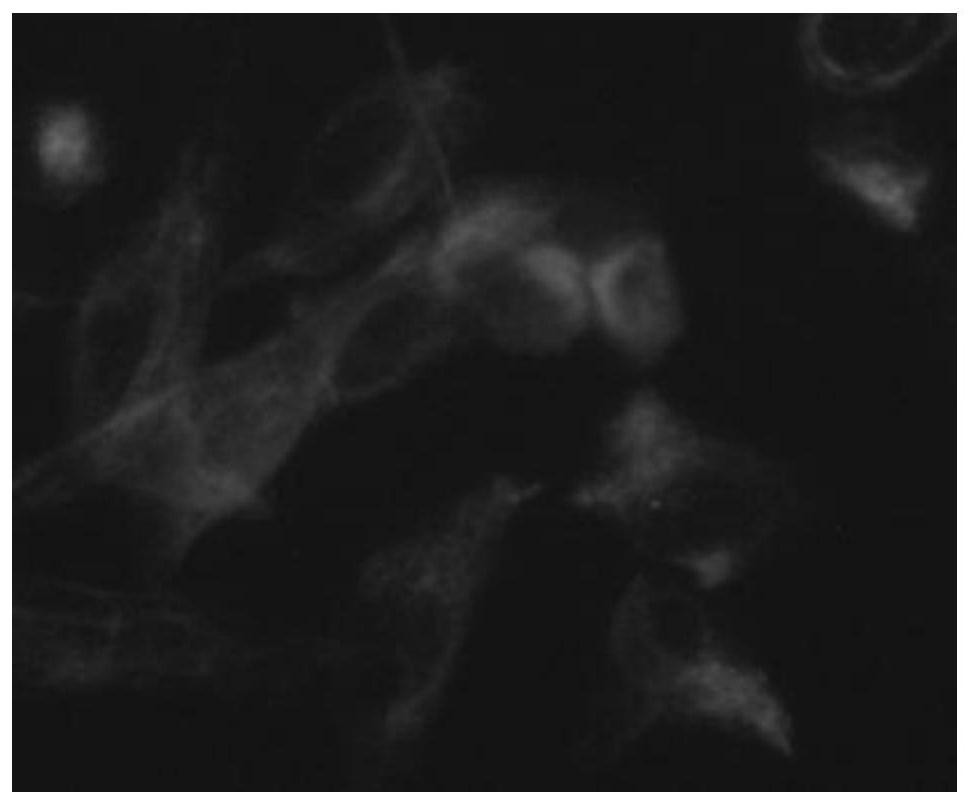
[0054] b. Cell immunofluorescence staining experiment:
[0055] Take the third-generation amniotic mesenchymal stem cells in a good growth state, adjust the cell concentration, and make cell slides. After the cells are full, fix them with 4% paraformaldehyde, do immunofluorescence staining, and observe under a fluorescent microscope to detect whether the cells contain Vimentin.
[0056] c. In vitro multi-lineage differentiation experiment:
[0057] After subculture, human amniotic mesenchymal stem cells were cultured in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com