IgG antigen epitope and application thereof as target
An antigenic epitope and site technology, applied in the field of tumor diagnosis and treatment in immunology, can solve the problem of inability to prepare diagnostic or therapeutic drugs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
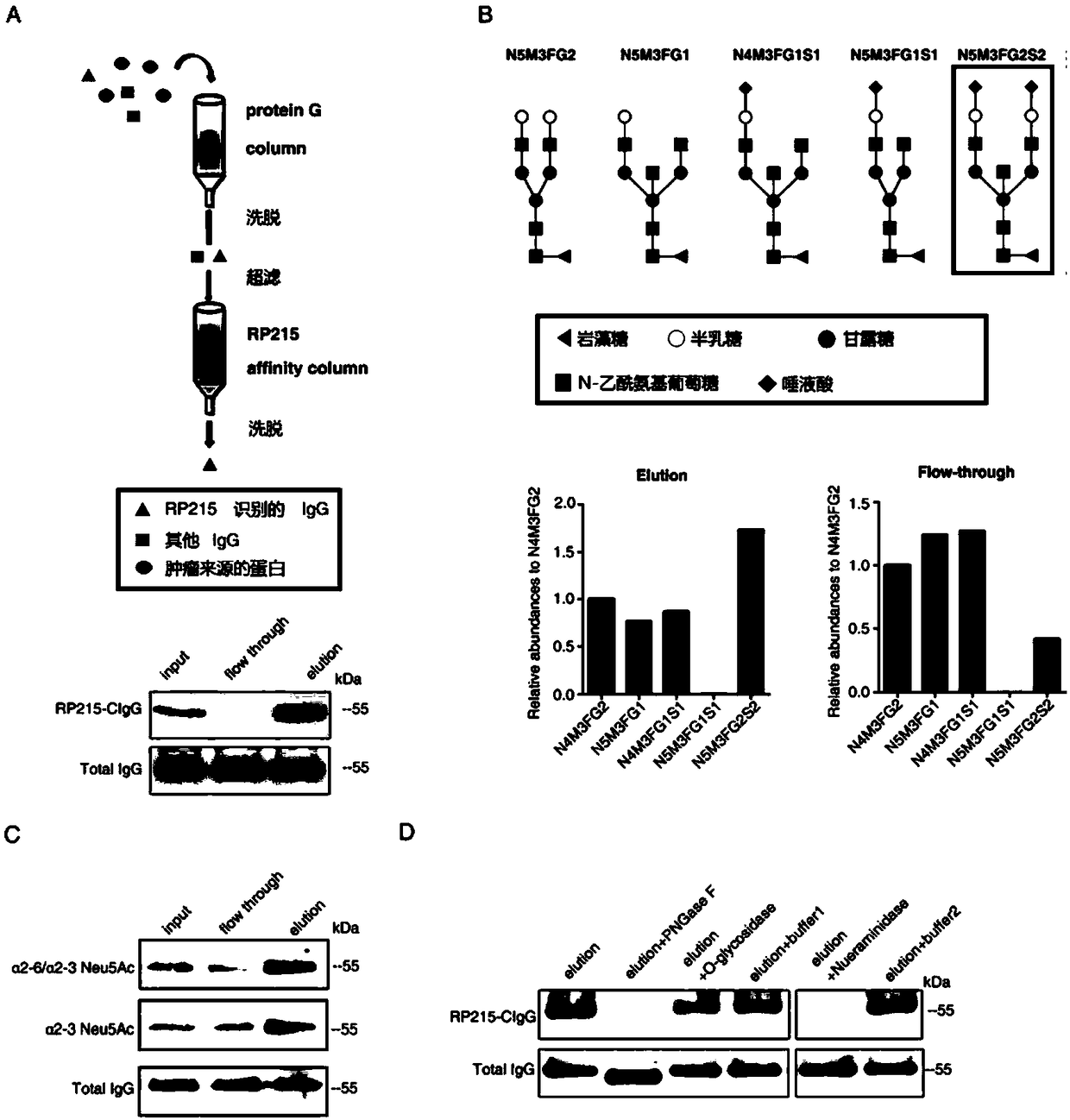
[0049] Example 1 Determination of the functional structure of RP215-IgG
[0050] 1. Purify IgG from cancer tissue
[0051] (1) Use protein G-Sepharose 4Fast Flow (GE healthcare, USA) to purify tumor IgG in advance from non-small cell lung cancer tissues, breast cancer or ovarian cancer tissues, or PDX tumor models established by lung squamous cell carcinoma (LSCC). The purpose of using the PDX tumor model is to exclude the influence of IgG derived from peripheral blood.
[0052] (2) Purify RP215-IgG with RP215 affinity column.
[0053] RP215 affinity column preparation method: monoclonal antibody RP215 is coupled with Sepharose 4FastFlow (GE Healthcare, USA) activated by CNBr, reference Picture 1-1 A. According to the instructions, proceed as follows: ① After 330 mg CNBr-sepharese 4B is activated by 1 mM hydrochloric acid, use coupling buffer (0.1M NaHCO 3 , 0.5M NaCl, pH8.3) balance. ② Dissolve 5 mg of RP215 antibody in coupling buffer, add it to activated CNBr agarose gel packing a...
Embodiment 2
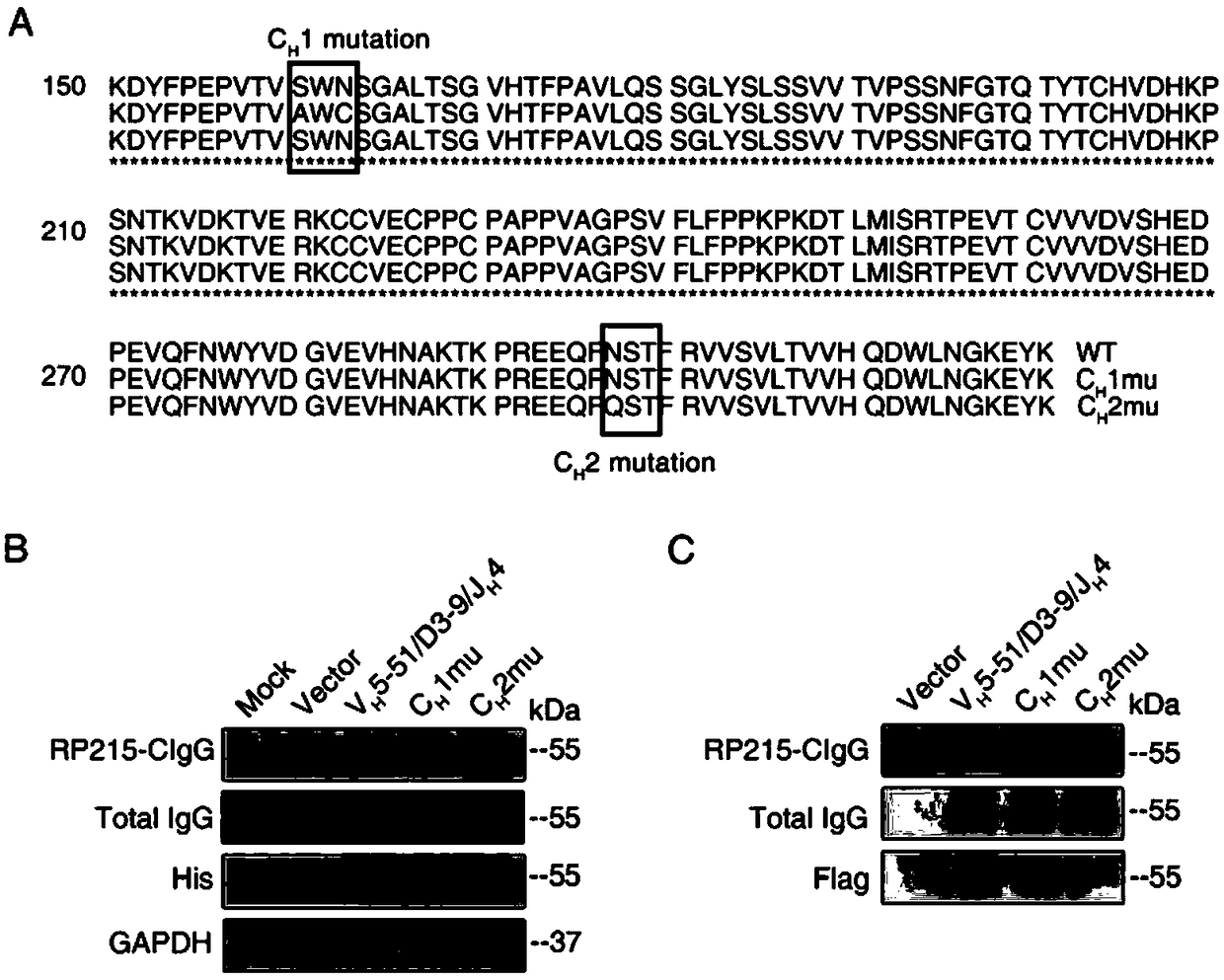
[0079] Example 2 IgG epitope is used as a target to identify non-B cell-derived IgG, which has the ability to promote tumor cell proliferation, migration and invasion
[0080] Example 1 confirmed that the unique epitope of IgG from non-B cells can be specifically recognized by RP215. Therefore, the epitope is used as the specific recognition site and RP215 is used to detect IgG from non-B cells ( RP215-IgG) function.
[0081] LSCC cell line: A549 (adenocarcinoma human alveolar basal epithelial cells), Calu-3 (human lung adenocarcinoma cells), NCI-H1299 (human lung cancer cells), NCI-H520 (human lung cancer cells), SK-MES-1 ( Human lung squamous cell carcinoma).
[0082] We first detected the expression of RP215-IgG in the above LSCC cell lines, and found that RP215-IgG that LSCC cell lines can express and secrete is located on the cell surface and ECM (see diagram 2-1 ).
[0083] When siRNA targeting the heavy chain constant region in NCI-H520 cells and SK-MES-1 cells down-regulates...
Embodiment 3
[0087] Example 3 Sialyltransferase ST3GAL4 participates in the sialylation of RP215-IgG
[0088] The biosynthesis of sialylated oligosaccharide sequences is catalyzed by a family of enzymes called sialyltransferases, and each sialyltransferase has its specific substrate.
[0089] Previous studies have shown that the sialic acid linked to the N-glycan at the classical N-glycosylation site (Asn297) is mediated by the sialyltransferase ST6GAL-1, and the sialic acid and N-glycan β-D -Galactopyranosyl (Gal) residues are linked in an α2,6-way.
[0090] We have determined that the sialic acid linked to the N-glycan at the non-canonical N-glycosylation site (Asn162) is linked to the β-D-galactopyranosyl (Gal) residue via MALI, so three saliva Acid transferases ST3GAL3, ST3GAL4, ST3GAL6 (involved in ST3β-galactoside α-2,3-linkage), and ST6GAL1 (as a control) were candidates for further screening.
[0091] In order to determine which sialyltransferase is involved in the synthesis of RP215-IgG ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com