Exosome, method for preparing same and application of exosome to preparing medicines for skin superficial tumor
A technology of exosomes and drugs, applied in the field of biomedicine, can solve the problems of lack of preparation methods to treat superficial tumors, achieve efficient and rapid preparation, solve body inflammation, and reduce immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
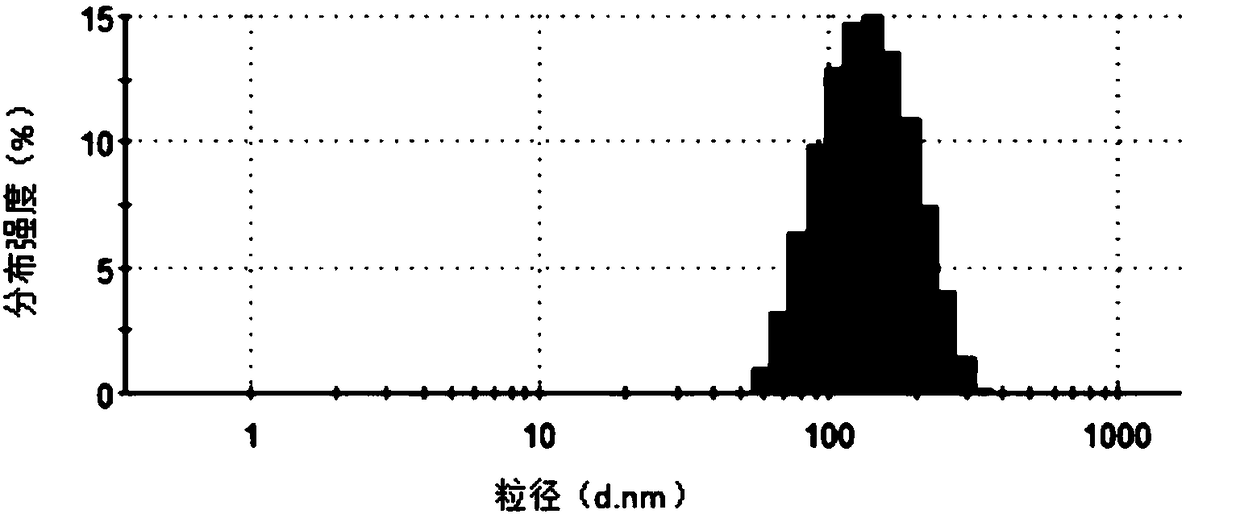
[0039] The frozen and transfected Escherichia coli were thawed overnight. The collected culture fluid was centrifuged at 5000g for 5 minutes at 4°C to remove dead cells and large debris. Filter the supernatant with a 0.22 μm filter membrane to further remove impurities such as bacteria. Transfer the supernatant to a sterile ultracentrifuge tube, centrifuge at 100,000 g for 2 hours at 4°C, and discard the supernatant. Wash with PBS, and ultracentrifuge at 100,000 g for 2 hours at 4°C, and the obtained precipitates are exosomes. Depending on the volume of the initially collected culture medium, add physiological saline for injection to resuspend as appropriate, use a BCA kit to detect the total protein concentration, and store in -80°C.
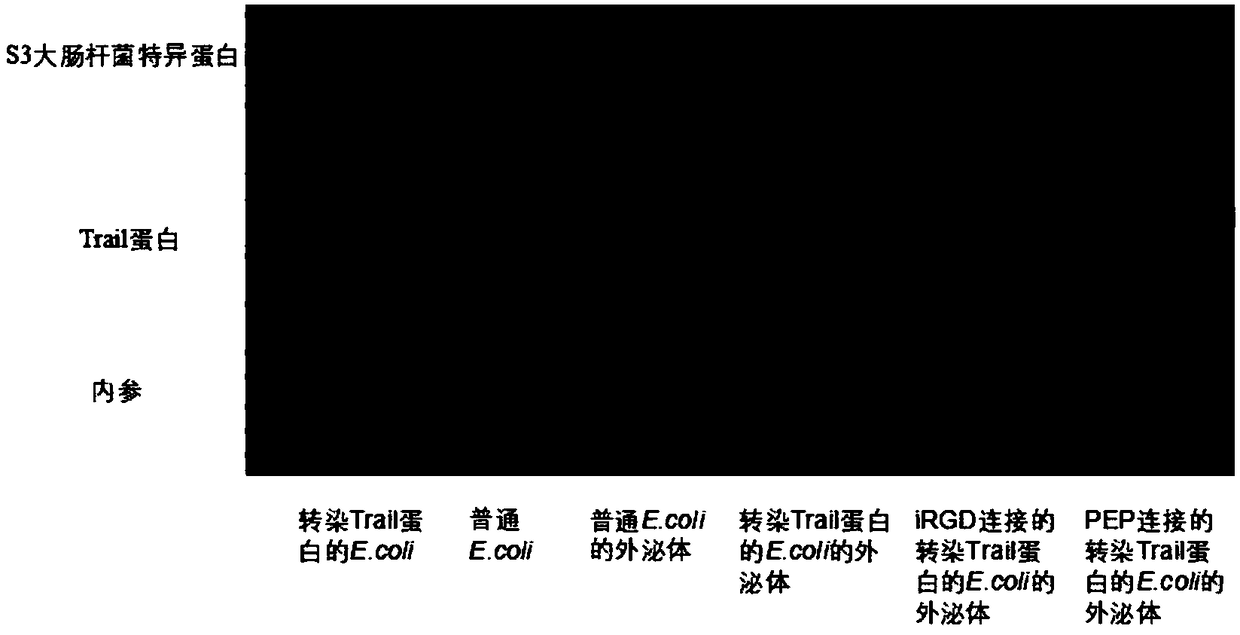
[0040] Add lysozyme (2mg / mL) to the exosomes, place in a shaker at 33°C, shake at 100rpm for 1h. Then add the polypeptide iRGD (25 μg / mL) and the polypeptide PEP (25 μg / mL) targeting melanoma respectively, wherein, the polypeptide iRGD is th...
Embodiment 2
[0042] The frozen and transfected Escherichia coli were thawed overnight. The collected culture fluid was centrifuged at 5000g for 5 minutes at 4°C to remove dead cells and large debris. Filter the supernatant with a 0.22 μm filter membrane to further remove impurities such as bacteria. Transfer the supernatant to a sterile ultracentrifuge tube, centrifuge at 100,000 g for 2 hours at 4°C, and discard the supernatant. Wash with PBS, and ultracentrifuge at 100,000 g for 2 hours at 4°C, and the obtained precipitates are exosomes. Depending on the volume of the initially collected culture medium, add physiological saline for injection to resuspend as appropriate, use a BCA kit to detect the total protein concentration, and store in -80°C.
[0043] Add lysozyme (1mg / mL) to the exosomes, place in a shaker at 20°C, shake at 80rpm for 1h. Then, the melanoma-targeted polypeptide iRGD (10 μg / mL) and the polypeptide PEP (10 μg / mL) were added respectively, placed in a shaker at 20° C.,...
Embodiment 3
[0045] The frozen and transfected Escherichia coli were thawed overnight. The collected culture fluid was centrifuged at 5000g for 5 minutes at 4°C to remove dead cells and large debris. Filter the supernatant with a 0.22 μm filter membrane to further remove impurities such as bacteria. Transfer the supernatant to a sterile ultracentrifuge tube, centrifuge at 100,000 g for 2 hours at 4°C, and discard the supernatant. Wash with PBS, and ultracentrifuge at 100,000 g for 2 hours at 4°C, and the obtained precipitates are exosomes. Depending on the volume of the initially collected culture medium, add physiological saline for injection to resuspend as appropriate, use a BCA kit to detect the total protein concentration, and store in -80°C.
[0046] Add lysozyme (8mg / mL) to the exosomes, place in a shaker at 33°C, shake at 200rpm for 3h. Then add peptide iRGD (30 μg / mL) and peptide PEP (30 μg / mL) targeting melanoma respectively, place in a shaker at 33 °C, shake at 200 rpm for 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com