Method for preparing high-purity hinokitiol and coordination complex thereof
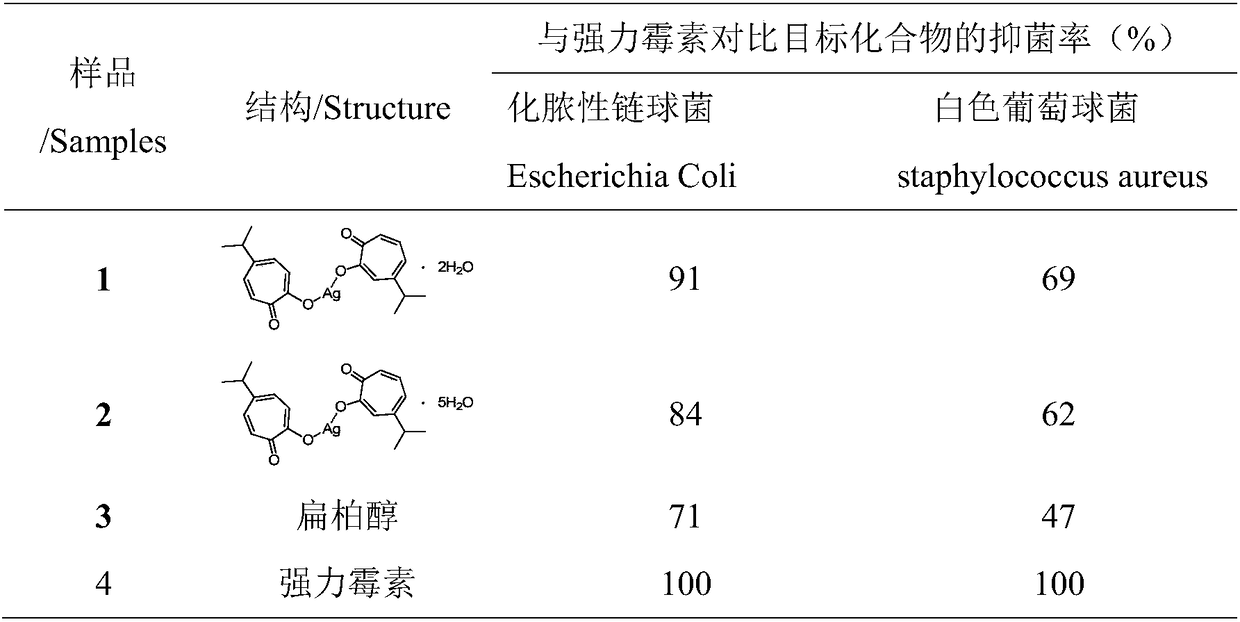
A high-purity technology for hinokitiol, which is applied in the field of preparation of high-purity hinokitiol and its complexes, can solve the problems of difficult to eliminate isomer by-products, cannot be industrially produced, and takes a long time to generate, and achieves excellent inhibitory effect and savings Production cost, effect of reducing the use of organic reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
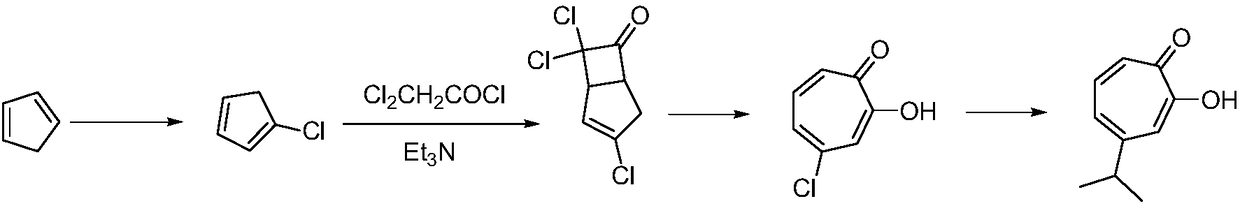
[0023] In order to overcome the defects in the synthesis of hinokitiol described in the background technology, one of the purposes of the present invention provides a method for preparing high-purity hinokitiol, comprising the following steps:
[0024] (1) Add cyclopentadiene to water at a temperature of 0-5°C, react with sodium hydroxide, N-chlorosuccinimide, and ether, filter the reaction solution with suction, add hydrochloric acid to the reaction solution, and adjust the pH The value is 6 to 7. After decompressing to distill off the ether, the reaction solution is extracted with ethyl acetate and concentrated to obtain 1-chlorocyclopentadiene;
[0025] (2) Add 1-chlorocyclopentadiene to water, keep the solution temperature at 0°C, and react with dichloroacetyl chloride, barium hydroxide, and chloroform to obtain 1-chlorocyclopentadiene-4,4-dichlorocyclobutane 5-keto;
[0026] (3) Add 1-chlorocyclopentadiene 4,4-dichlorocyclobutane 5-one to a mixed solvent of acetic acid, ...
Embodiment 1
[0041]Add 66g (1mol) of cyclopentadiene into 330mL of water, set the reaction temperature to 0-5°C, slowly add 150g of sodium hydroxide solution with a concentration of 40% dropwise, stir for 30min after the dropwise addition, rise to room temperature, and divide Add 140g of N-chlorosuccinimide in four batches, 35g in each batch. After adding the last batch of NCS, keep stirring at 40°C for 30 minutes. TLC monitors the complete reaction of the raw materials. After cooling down to 10°C, pour into the reaction solution Add 460g of diethyl ether, a white solid precipitates, filter the reaction solution with suction, add a certain amount of hydrochloric acid to the reaction solution, adjust the pH to 6-7, then distill the ether under reduced pressure, the diethyl ether can be reused, and extract the reaction solution with ethyl acetate , 96 g of 1-chlorocyclopentadiene was obtained after concentration, and the yield was 96%.
Embodiment 2
[0043] Add 66g (1mol) of cyclopentadiene into 330mL of water, set the reaction temperature to 0-5°C, slowly add 100g of sodium hydroxide solution with a concentration of 40% dropwise, stir for 30min after the dropwise addition, rise to room temperature, and divide Add 140g of N-chlorosuccinimide in four batches, 35g in each batch. After adding the last batch of NCS, keep stirring at 40°C for 30 minutes. TLC monitors the complete reaction of the raw materials. After cooling down to 10°C, pour into the reaction solution Add 460g of diethyl ether, a white solid precipitates, filter the reaction solution with suction, add a certain amount of hydrochloric acid to the reaction solution, adjust the pH to 6-7, then distill the ether under reduced pressure, the diethyl ether can be reused, and extract the reaction solution with ethyl acetate After concentration, 74 g of 1-chlorocyclopentadiene was obtained, and the yield was 74%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com