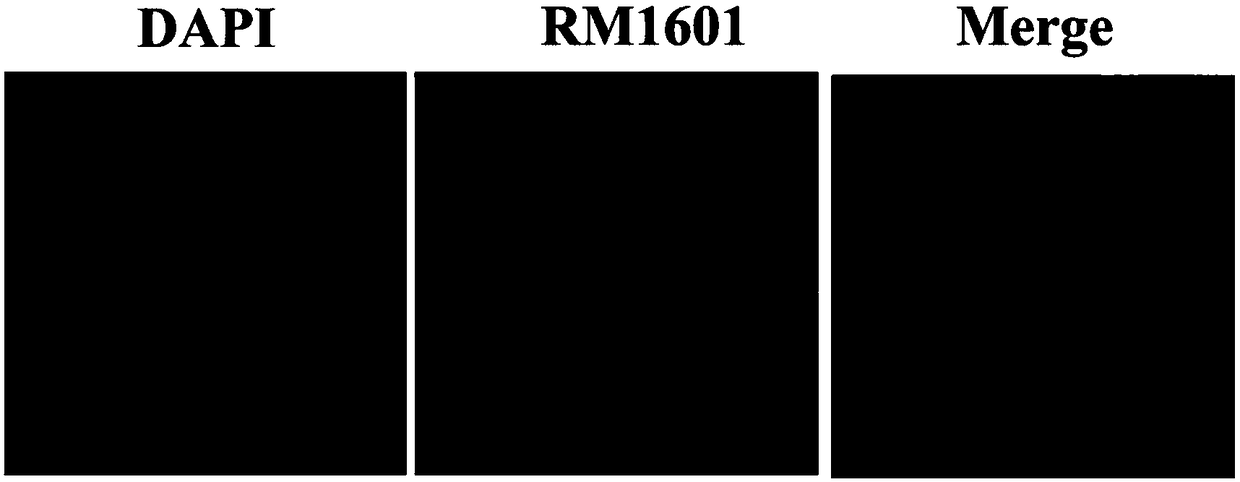
Alkynyl-containing ruthenium complex as well as synthesis method and application thereof
A technology of ruthenium complexes and synthesis methods, applied in the directions of ruthenium organic compounds, compounds containing elements of Group 8/9/10/18 of the periodic table, organic compounds/hydrides/coordination complex catalysts, etc. Cells do not have obvious cytotoxicity and other problems, and achieve significant anti-tumor activity, promote transmembrane absorption, and increase drug efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The synthesis of embodiment 1 ligand Br-DPPZ
[0075]
[0076] Weigh 315.16mg (1.5mmol) of o-phenanthroline 5,6-dione, 232.48mg (1.25mmol) of 4-bromo-o-phenylenediamine, add 20ml of absolute ethanol to a 30ml quartz tube, stir for 5 minutes, microwave React at 95°C for 3 minutes. After the reaction, cool to room temperature, add 30ml of water, a yellow-white solid precipitates, filter with suction, wash with water to obtain a filter cake, and dry in a vacuum oven at 50°C. Pure chloroform was dissolved, and pure chloroform was packed into a column, and the eluent was eluted with pure chloroform:dehydrated ethanol=100:1, and the main yellow band was collected, and the yellow solid was spin-dried under reduced pressure, and dehydrated alcohol:chloroform= 3:2 solvent for recrystallization. Yield: 80%.
Embodiment 2
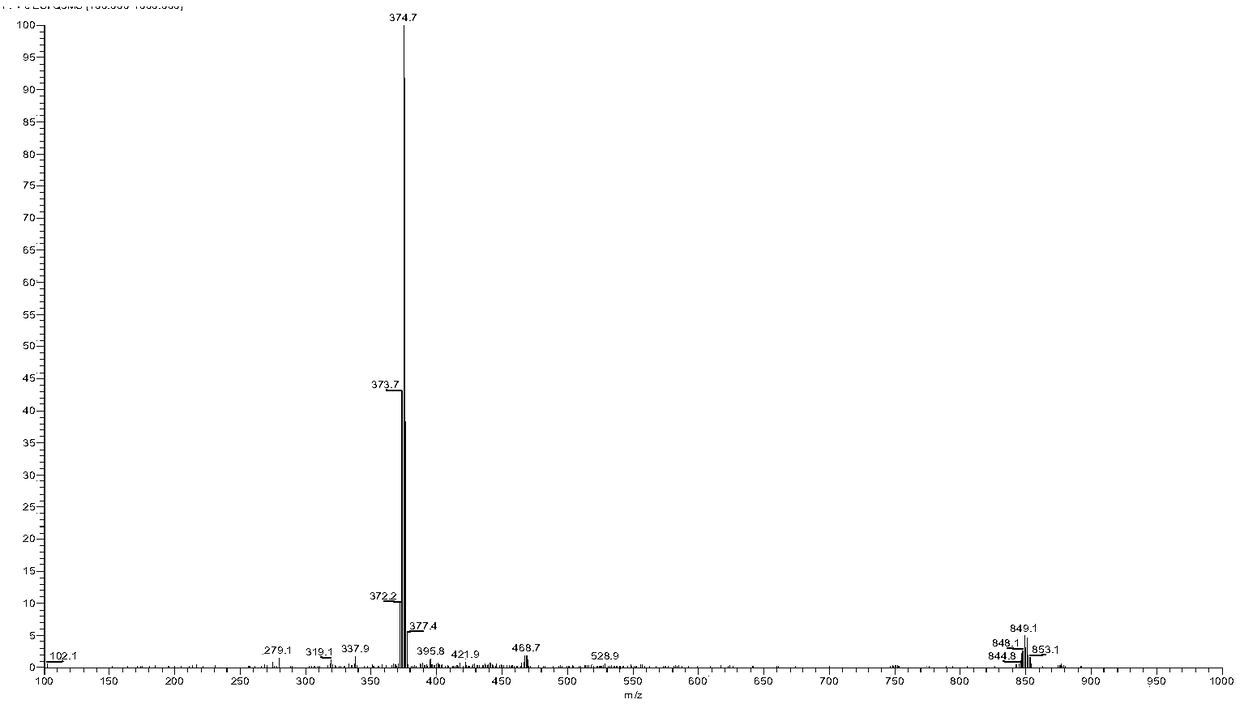
[0077] Embodiment 2 [Ru(bpy) 2 (Br-DPPZ)] 2+ Synthesis
[0078]
[0079] In the 50ml three-neck bottle, put into appropriate ratio (1.5:1) of Br-DPPZ, [Ru(bpy) 2 Cl 2 ]·2H 2 O and ethylene glycol and water mixed solvent (V 乙二醇 :V 水 =9:1) 30ml, heat to reflux for 6 hours (T=120°C), stop the reaction, cool to room temperature, add 80ml of water to dilute, filter to obtain an orange-red clear filtrate, add excess NaClO to the filtrate 4 , producing a large amount of orange-red precipitate, which was filtered by suction and dried in a vacuum desiccator to obtain an orange-yellow crude product. The crude product was dissolved with a small amount of acetonitrile, purified and separated by a neutral alumina column, loaded with acetonitrile, and the main red component was washed with acetonitrile in an appropriate proportion, spin-dried under reduced pressure and then dried in a vacuum desiccator to obtain red crystals with a yield of 80% %.
Embodiment 3
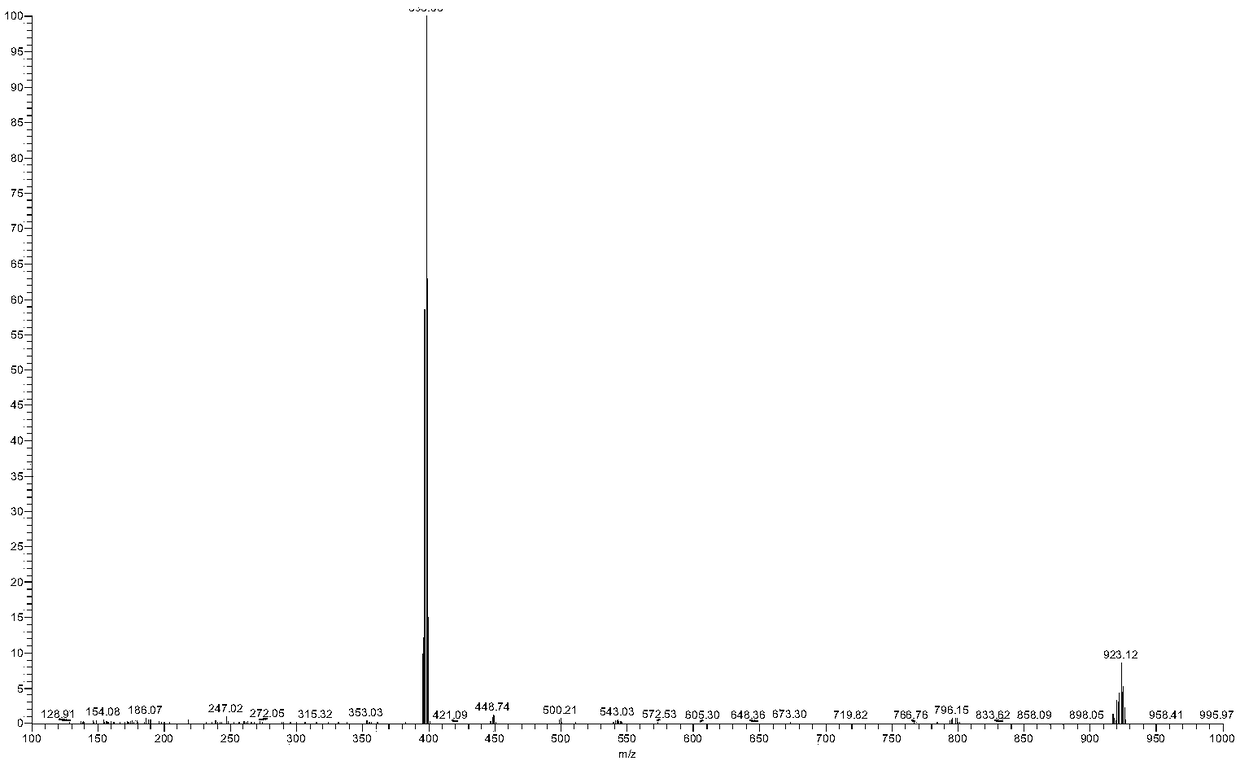
[0080] Synthesis of Example 3 RM1601
[0081]
[0082] Weigh [Ru(bpy) 2 (BrDPPZ)] 2+ (125mg, 0.125mmol) in a 10ml microwave reaction tube (stirring bar was added to the tube), add 5ml of anhydrous acetonitrile supernatant to dissolve completely, under the protection of nitrogen, add appropriate amount of triethylamine (70μL, 0.501μmol) and phenylacetylene (70μL, 0.637 μmol), and then quickly add the catalyst Pd (PPh 3 ) 2 Cl 2 (17.6mg), CuI (20mg); after airtight, put into a microwave reactor, and microwave-assisted heating at 140°C for 30min. Cool to room temperature after the reaction, filter, and purify the filtrate through a neutral alumina column, elute with pure acetonitrile, and separate 4 bands, 1 band is light yellow, 2 bands are dark reddish brown, 3 bands are light yellow bands, and 4 bands are dark reddish brown . Pure acetonitrile was eluted, 1 band and 2 bands were collected, 3 bands were collected for elution with acetonitrile:methanol=50:1, which was t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com