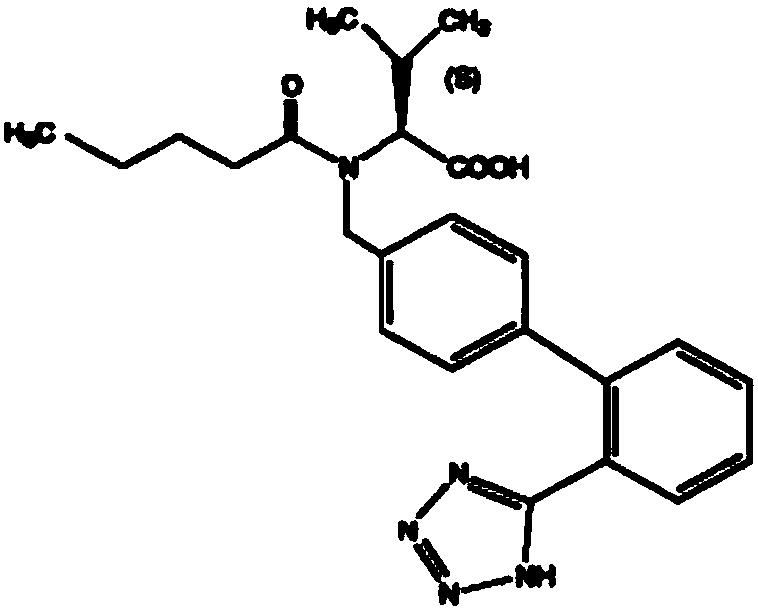
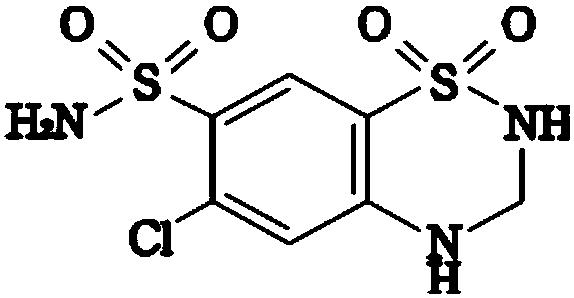
Valsartan/hydrochlorothiazide tablet and preparation method thereof
A technology for chlorothiazide tablets and hydrochlorothiazide is applied in the field of medicine and can solve the problems of increased degradation products, low in vitro dissolution rate, influence on drug absorption and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, preparation valsartan / hydrochlorothiazide sheet
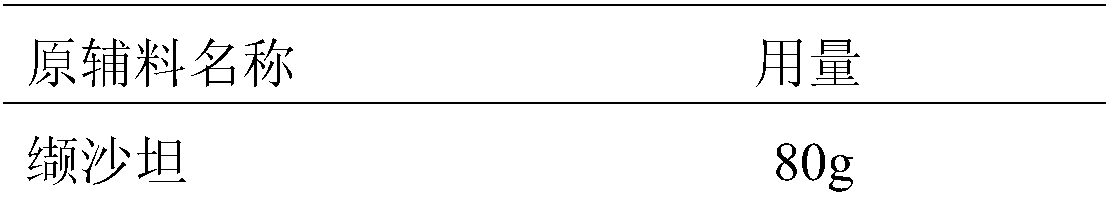
[0047] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
[0048] Prescription composition:
[0049]
[0050]
[0051] Control the ambient humidity RH≤40%.
[0052] The preparation method comprises the following steps:
[0053] 1) Grinding hydrochlorothiazide to obtain hydrochlorothiazide powder (particle size range D90≤130 μm), passing crospovidone XL through a 40-mesh sieve, and magnesium stearate (additional) passing through a 80-mesh sieve;
[0054] 2) Weigh valsartan, crospovidone XL, copovidone S630 (internal addition), magnesium stearate (internal addition), hydrochlorothiazide, colloidal silicon dioxide, microcrystalline cellulose PH102 and mix in wet method Mix in the granulator for 2 minutes (stirring 3r / s, shearing 3r / s) and pass through a 40-mesh sieve to disperse;
[0055] 3) Pre-mixing: put the ...
Embodiment 2
[0062] Embodiment 2, preparation valsartan / hydrochlorothiazide sheet
[0063] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
[0064] Prescription composition:
[0065]
[0066] The preparation method is basically the same as in Example 1.
Embodiment 3
[0067] Embodiment 3, preparation valsartan / hydrochlorothiazide sheet
[0068] The present embodiment provides the prescription and preparation method for preparing 1000 valsartan / hydrochlorothiazide tablets, as follows:
[0069] Prescription composition:
[0070]
[0071]
[0072] The preparation method is basically the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com