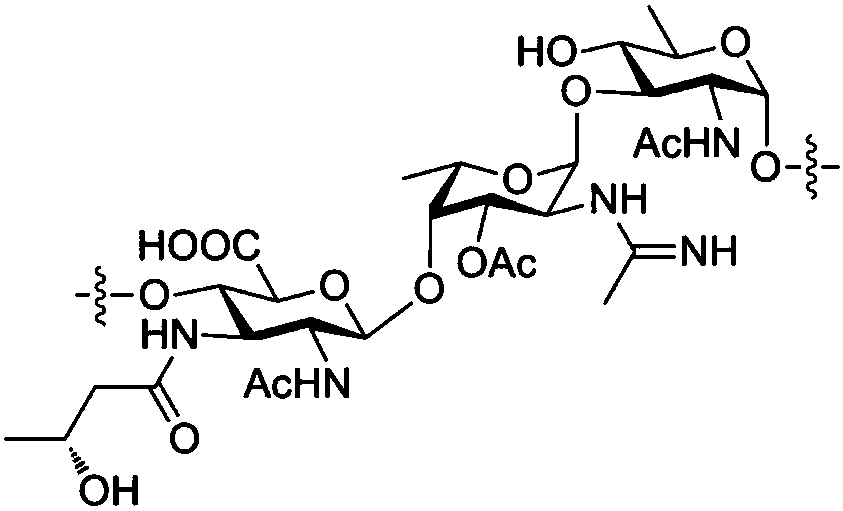
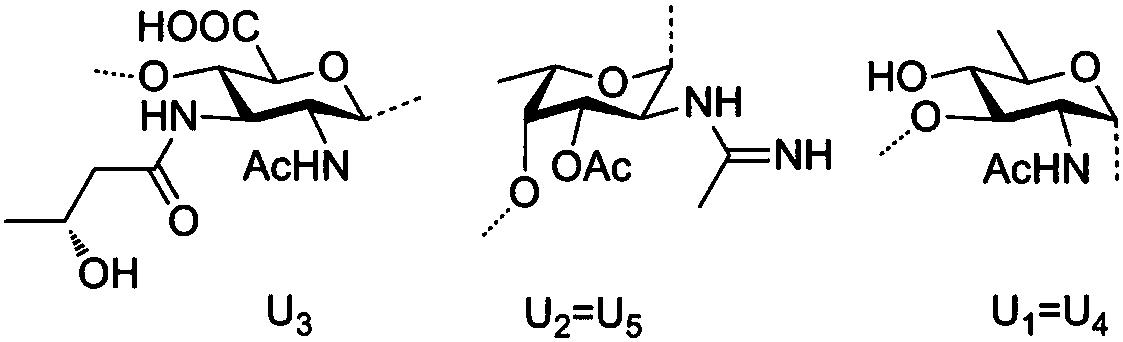
Chemical synthesis method of plesiomonas shigelloides O51 serotype O-antigen oligosaccharide
A kind of technology of necromonas and serotypes, applied in the field of chemistry, can solve the problem of unreported chemical synthesis and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Synthesis of allyl 3,4,6-tri-O-acetyl-2-deoxy-2-trichloroacetylamino-α-D-glucopyranose (1*)
[0132] The reaction equation is as Figure 20 shown;
[0133] Under argon protection, 1,3,4,6-tetra-O-acetyl-2-deoxy-2-trichloroacetylamino-β-D-glucopyranose (M. Virlouvet et al., Adv.Synth .Catal.2010,352,2657-2662) (27g, 0.055mol) was dissolved in anhydrous dichloromethane (130mL), and activated Molecular sieves and allyl alcohol (18.7 mL, 0.274 mol). After cooling to -5°C, boron trifluoride diethyl ether (70 mL, 0.548 mol) was added dropwise. The reaction solution was stirred at 0° C. for 30 min, and then raised to room temperature for 71 hours. After the reaction, the reaction solution was poured onto 200 g of crushed ice, and the organic phase obtained after filtration with diatomaceous earth was extracted with water, saturated sodium bicarbonate solution, and saturated saline respectively, and the obtained organic phase was concentrated after dehydration by sodium su...
Embodiment 2
[0135] Synthesis of Allyl 4,6-O-benzylidene-2-deoxy-2-trichloroacetylamino-α-D-glucopyranose (2*)
[0136] The reaction equation is as Figure 20 shown;
[0137] Compound 1* (50 g, 0.102 mol) was dissolved in methanol (800 mL), added with sodium methoxide (2.8 g, 0.051 mol) and stirred at room temperature for 5 hours. After the reaction, the reaction solution was neutralized with Amberlite IR 120 cation exchange resin, filtered and concentrated to obtain a white solid which was the target 3,4,6-trihydroxy sugar (37.1 g, 0.102 mol, quant.).
[0138] Trihydroxy sugar (71 g, 0.195 mol) was further dissolved in 300 mL of anhydrous DMF, benzaldehyde dimethyl acetal (35 mL, 0.234 mol) and p-toluenesulfonic acid (4.45 g, 0.023 mol) were added. After the reaction solution was reacted at 60°C for 24 hours, use a rotary evaporator to distill off the methanol generated in the reaction under reduced pressure, and continue to react at 60°C. This operation was repeated. After the reaction...
Embodiment 3
[0140] Synthesis of Allyl 4,6-O-benzylidene-3-O-trifluoromethanesulfonyl-2-trichloroacetamido-2-deoxy-α-D-glucopyranose (3*)
[0141] The reaction equation is as Figure 20 shown;
[0142] Compound 2* (53.1g, 0.117mol) was dissolved in anhydrous dichloromethane / pyridine mixture (660mL, 7:1, v / v), and after cooling down to -20°C, trifluoro A solution of methanesulfonic anhydride (40 mL, 0.234 mol) in dichloromethane (150 mL) was gradually warmed to 10°C over 2 hours. After the reaction, the reaction solution was diluted with dichloromethane, and extracted with 1M HCl solution, saturated sodium bicarbonate, water and saturated brine in sequence. The organic phase was dehydrated by anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure at low temperature. The obtained crude product was purified by silica gel column chromatography (petroleum ether: ethyl acetate, 5:1 to 2:1, v / v) to obtain yellow syrup Product 3* (66.5g, 0.114mol, 98%). [α] D 20 =...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com