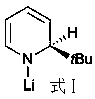
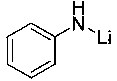
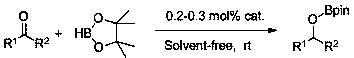Application of anilino lithium to catalysis of ketone and borane to generate hydroboration reaction
A technology of anilinolithium and hydroboration, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of weakening carbonyl nucleophilic addition activity and weakening carbonyl Carbon positive, harsh reaction conditions and other issues, to achieve the effect of simple and controllable reaction, good universality, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Lithium anilide catalyzes the hydroboration reaction of methyl isopropyl ketone and pinacol borane
[0022] In the reaction flask that has been dehydrated and deoxygenated, add 40ul tetrahydrofuran solution (0.05M) of lithium anilide (0.2 mol% dosage) under the protection of argon, then add 0.1596 mL of borane with a syringe, mix well, and then add 0.1072 mL of borane with a syringe. mL of methyl isopropyl ketone, the mixture was stirred at room temperature, and after 30 min of reaction, the NMR yield was 99%, after which a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate . 1 H NMR (400 MHz, CDCl 3 ) δ 3.94 (p, J = 6.2 Hz, 1H, OCH), 1.66 (dq, J = 13.5, 6.8 Hz, 1H, CH 3 CH), 1.25 (s, 12H, CH 3 ), 1.14 (d, J = 6.3 Hz, 3H, CH 3 ), 0.91-0.85 (m, 6H,CHCH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 82.34 (OC), 75.40 (OCH), 34.25 (OCHCH), 24.50 (d, J = 7.4 Hz, CH 3 ), 19.30 (CH 3...
Embodiment 2
[0023] Example 2: Lithium anilide catalyzes the hydroboration reaction of 4-heptanone and pinacol borane
[0024] In the reaction flask that has been dehydrated and deoxygenated, add 60ul tetrahydrofuran solution (0.05M) of lithium anilide (0.3 mol% dosage) under the protection of argon, then add 0.1596 mL borane with a syringe, mix well, and then add 0.1398 mL with a syringe mL4-heptanone, and the mixture was stirred at room temperature. After 40 min of reaction, the NMR yield was 99%. After that, a small amount of tetrahydrofuran and excess borane were removed under reduced pressure to obtain the corresponding pinacol borate. 1 H NMR (400 MHz, CDCl 3 ) δ4.03 (td, J = 8.0, 3.9 Hz, 1H, CH), 1.51 – 1.31 (m, 8H, CH 2 ), 1.24 (s, 12H,CH 3 ), 0.90 (t, J = 7.1 Hz, 6H, CH 3 ). 13 C NMR (101 MHz, CDCl 3 ) δ 81.82 (OC), 73.55 (OCH), 38.18 (CH2), 23.97 (CH 3 ), 18.10 (CH 3 ), 13.48 (CH 3 ).
[0025] Replacing lithium anilide with the lithium amidide compound of formula I, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com