A method for detecting 1-(2-hydroxyethyl)piperazine in dasatinib
A technology of dasatinib and hydroxyethyl, applied in the field of drug analysis, achieves high precision, simple operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Experimental materials and instrument conditions
[0053] Instrument: HPLC: Agilent 1260; Column: Thermo Acclaim TM C18, 5μm, 4.6×250 mm; flow rate: 1.0mL / min; column temperature: 30℃; injection volume: 20μl; running time: 25min; detection wavelength: 340nm. Inject the blank solution, the reference substance solution and the test solution into the liquid chromatograph respectively, record the chromatogram, and the chromatographic conditions are as follows: Chromatographic column: use octadecylsilane bonded silica gel as filler; Temperature: 30°C; Injection volume: 20μl; Running time: 25min; Detection wavelength: 340nm; Mobile phase: 0.5%wt triethylamine (adjust pH to 3 with phosphoric acid)-acetonitrile, carry out gradient elution, the gradient is as shown in the table 1.
[0054] Table 1 - Gradient Elution Conditions
[0055] time (min)
mobile phase A
mobile phase B
0
75
25
15
25
75
16
75
25
25
75
...
Embodiment 2
[0068] Embodiment 2 detection method system applicability test of the present invention
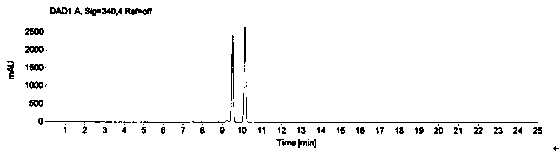
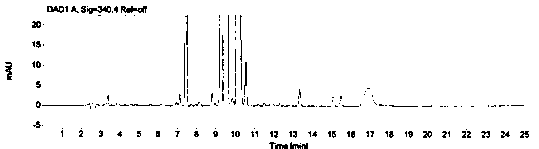
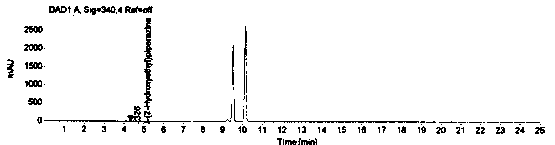
[0069] The system suitability is realized by measuring the RSD of the peak area of 1-(2-hydroxyethyl)piperazine in the reference solution for 5 points, and it is required that the peak area of 1-(2-hydroxyethyl)piperazine in the reference solution is 5 points RSD≤2.0%. Prepare blank solution and reference substance solution as described in Example 1, under the chromatographic conditions described in Example 1, enter 1 needle of blank solution, 5 needles of reference substance solution, obtain the chromatogram, as figure 1 , figure 2 , image 3 and Figure 4 , according to the formula conversion results are shown in the table below:
[0070] Table 2 - System Suitability Determination Results
[0071]
[0072] The conclusion is: the RSD of the peak area of 1-(2-hydroxyethyl)piperazine in the reference solution of 5 is 0.9%, which meets the requirement that the RSD in the prot...
Embodiment 3
[0073] Embodiment 3 Detection method specificity test of the present invention
[0074] The specificity of the method is achieved by studying peak identification and selectivity, requiring that the blank solvent does not interfere with the detection; dasatinib positioning solution has no interference with the detection; 1-(2-hydroxyethyl)piperazine in the selective solution The degree of separation from adjacent peaks is ≥1.5; in the selective solution, the recovery rate of 1-(2-hydroxyethyl)piperazine should be between 90.0% and 110.0%. Prepare blank solution, reference solution, 1-(2-hydroxyethyl)piperazine positioning solution, test solution, dasatinib positioning solution and selective solution as described in Example 1.
[0075] After the system is balanced, inject 1 needle of blank solution, 1 needle of 1-(2-hydroxyethyl)piperazine positioning solution, 1 needle of dasatinib positioning solution, and 3 needles of selective solution, and record the chromatogram, such as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com