Detection method of higenamine hydrochloride injection related substances
A technology of higenamine hydrochloride and injection, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of undetectable degraded impurities, etc., and achieve the effects of short time, high precision and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) Experimental materials and instrument conditions
[0079] Instrument: High Performance Liquid Chromatography: Agilent 1260; Chromatographic Column: CAPCELL PAK C18 MG (S-5) 4.6mm×250mm; Flow Rate: 1.0ml / min; Column Temperature: 30°C; Injection Volume: 20μl; Detection Wavelength: 284nm, mobile phase: A2: 0.05% EDTA disodium solution; mobile phase B2: methanol;
[0080]
[0081] (2) Experimental steps
[0082] Diluent: 0.05% EDTA disodium solution;
[0083] Blank solution 2: diluent;
[0084] Reference solution: Accurately weigh 25mg of higinarine hydrochloride reference substance, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, and shake well. Accurately measure 1.0ml, put it in a 100ml measuring bottle, add diluent to dilute to the mark, and shake well.
[0085] Sensitivity solution: Accurately measure 1.0ml of reference solution, put it in a 20ml measuring bottle, add diluent to dilute to the mark, and shake well.
[0086]...
Embodiment 2
[0107] Embodiment 2 detection method system applicability test of the present invention
[0108] The system suitability was checked at the beginning of the sequence; the system suitability was realized by measuring the repeatability of the reference solution, and the RSD% of the peak area of higenamine hydrochloride in the reference solution was less than or equal to 10.0%. At the same time, the signal-to-noise ratio of the main peak of the sensitivity solution is required to be greater than or equal to 10. Prepare blank solution 2, auxiliary material blank solution, reference solution, and sensitivity solution as described in Example 1. After the system is stable, run the system gradient blank once, and then enter blank solution 2 for 1 needle, auxiliary material blank solution for 1 needle, and refer to 5 needles of specific solution, 1 needle of sensitivity solution, and record the chromatogram.
[0109]
Embodiment 3
[0110] Embodiment 3 Detection method specificity test of the present invention
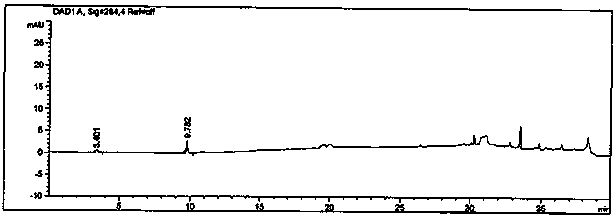
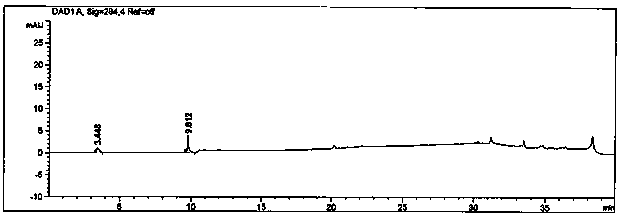
[0111] The specificity research of the method investigates the peak identification and selectivity, and the separation between the main peak and the adjacent impurity peaks is required to be ≥1.5, the signal-to-noise ratio (S / N) of the sensitivity solution is ≥10, and the blank solvent has no obvious interference to the detection. After the system is balanced, inject 1 needle of blank solution 2, 1 needle of reference solution, 1 needle of excipient blank solution, 1 needle of impurity A reference solution, 1 needle of impurity B reference solution, 1 needle of mixed reference solution, and test solution S1 needle, test solution P 1 needle, record the chromatogram, and obtain the specificity detection results as follows.
[0112]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com