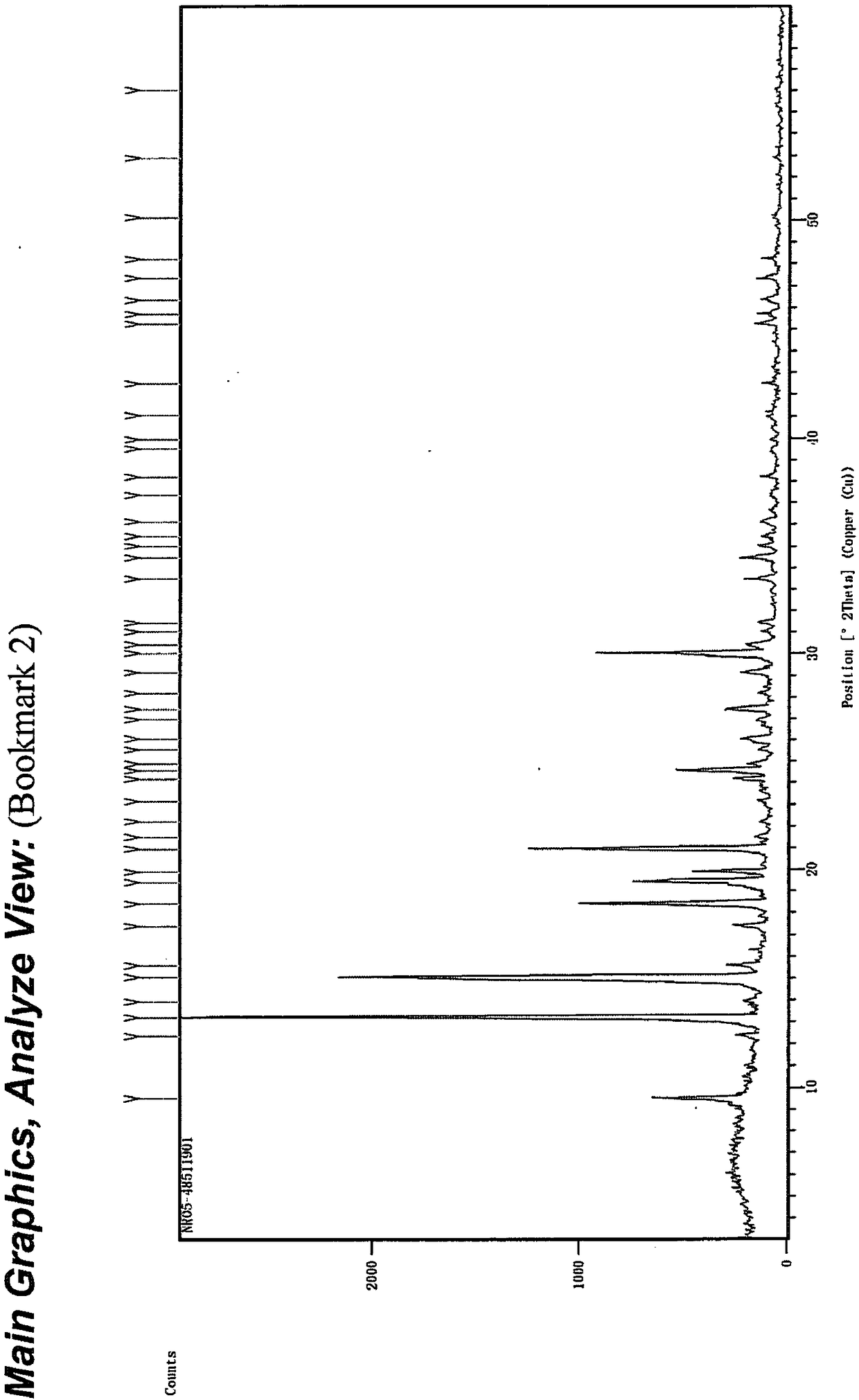
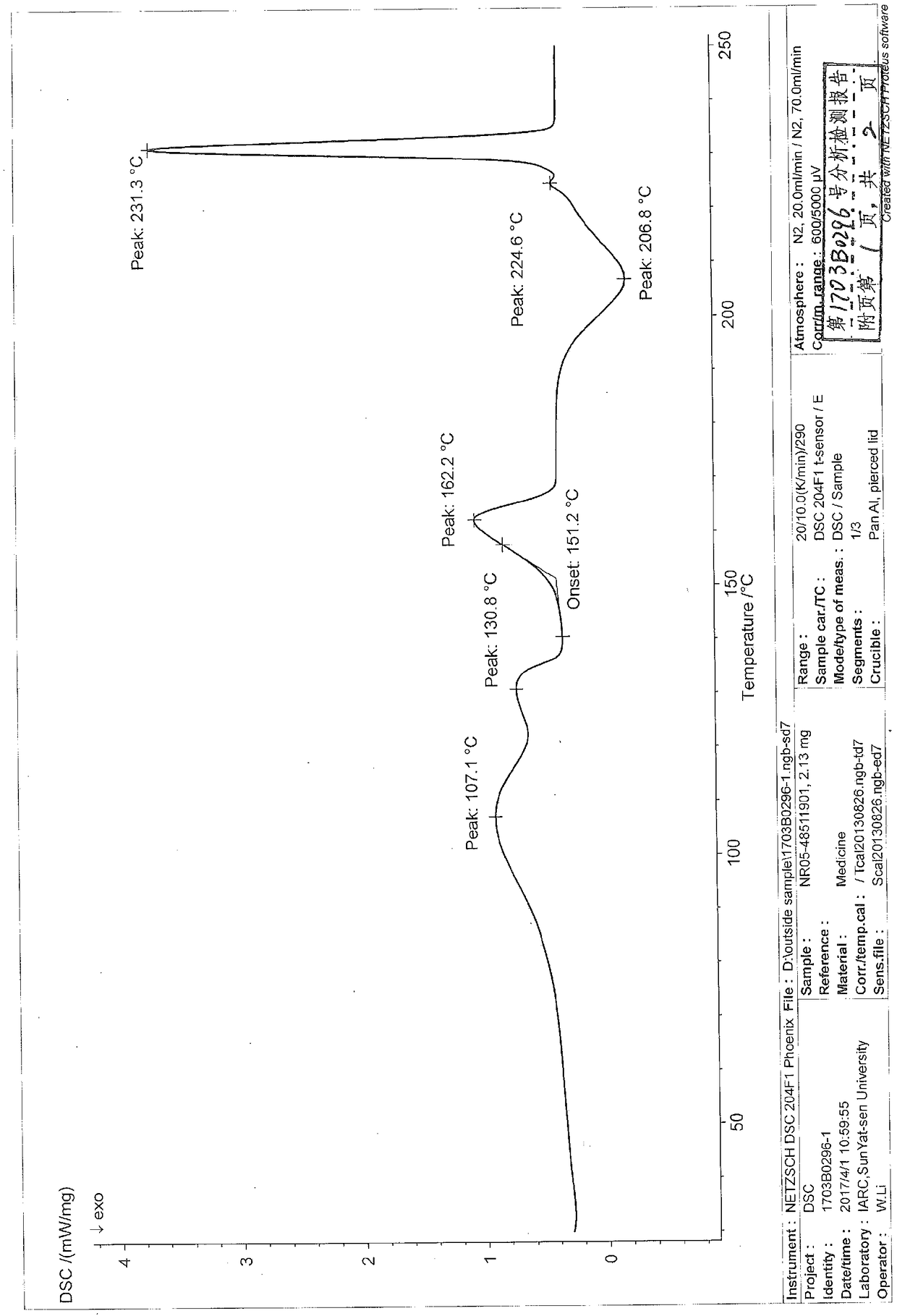
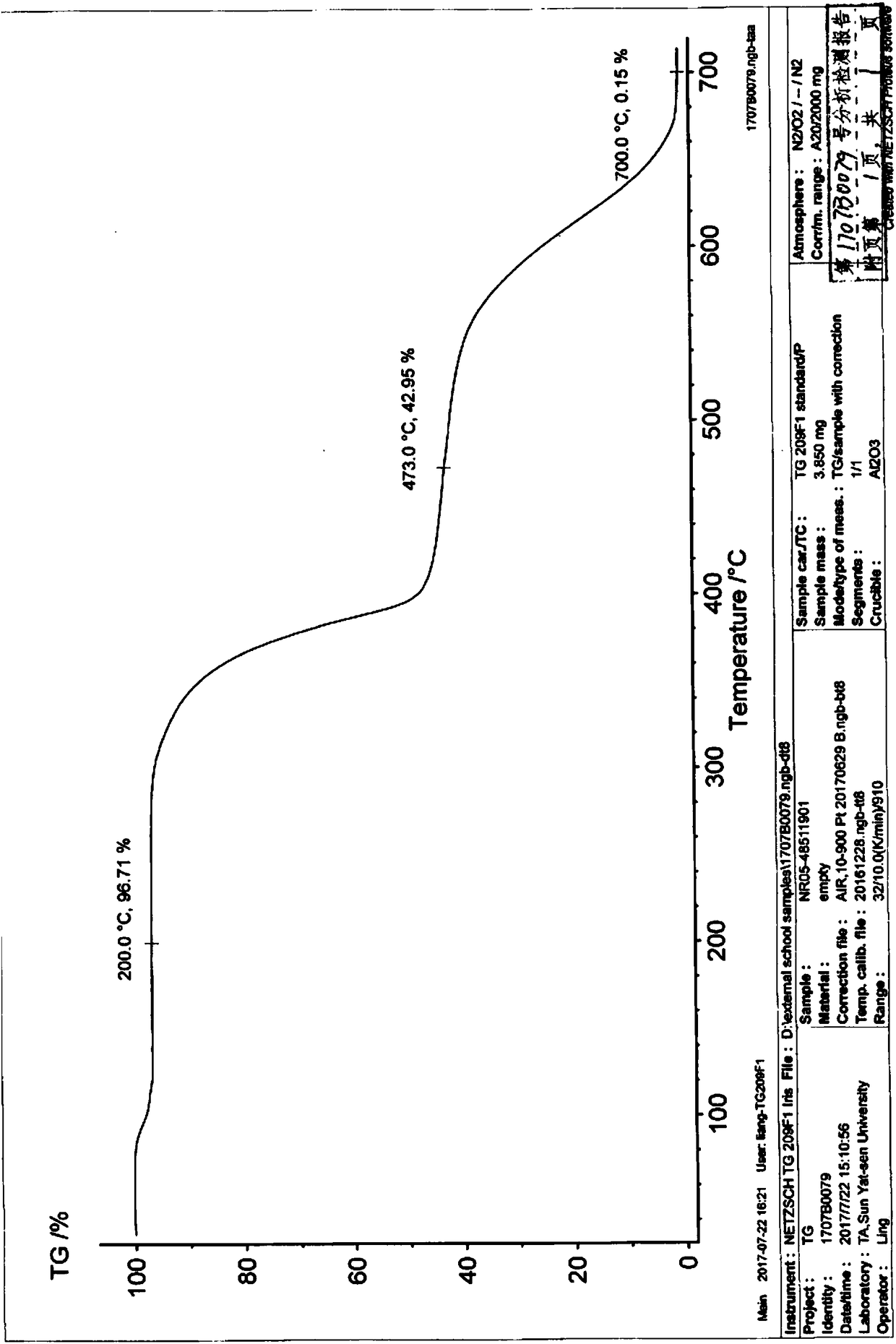
Niraparib p-toluene sulfonate hydrate crystal form and preparation method thereof
A technology of p-toluenesulfonate and monohydrate, applied in the field of chemical medicine, can solve the problems of loss of activity, DNA damage, cancer cell death, etc., and achieve the effect of low hygroscopicity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis method of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-carboxamide p-toluenesulfonate hydrate crystal form A
[0058] Add 120g of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-formyl p-toluenesulfonate monohydrate into the reaction flask, 980mL of ethanol, 250mL of water, nitrogen Stir and heat to 80°C under protection, the solid dissolves, slowly cool down to 20°C under stirring and stir at controlled temperature for 2 hours, filter, and vacuum-dry at 40°C to obtain 105g of Form A.
Embodiment 2
[0060] Synthesis method of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-carboxamide p-toluenesulfonate hydrate crystal form A
[0061] Add 100g of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-formyl methanesulfonate and 1000mL of water into the reaction flask, and control the temperature to 20°C-30°C. Under the protection of nitrogen, slowly add 50% (mass fraction) p-toluenesulfonic acid (70g) monohydrate solution, after the addition, control the temperature at 20°C and stir for 4 hours, filter and wash with water, and dry under vacuum at 40°C to obtain 100g of Form A.
Embodiment 3
[0063] Synthesis method of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-carboxamide p-toluenesulfonate hydrate crystal form A
[0064] Add 100g of 2-[4-((3S)-3-piperidinyl)phenyl]-2H-indazole-7-carboxyl, 750mL of ethanol, 250mL of water into the reaction flask and control the temperature to 20°C-30°C, nitrogen Slowly add 50% (mass fraction) of p-toluenesulfonic acid (75g) monohydrate solution under protection, after the addition is completed, stir at 20°C for 4 hours, filter, wash with water, and dry under vacuum at 40°C to obtain 110g of Form A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com