Method for constructing engineered artery blood vessel in vivo by taking melt-spun fibers as framework
A melt spinning, arterial blood vessel technology, applied in the field of medical devices, can solve the problems of easy formation of acute thrombus, body damage, vascular stenosis, etc., and achieve the effects of avoiding aneurysm formation, good biocompatibility, and reducing restenosis rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
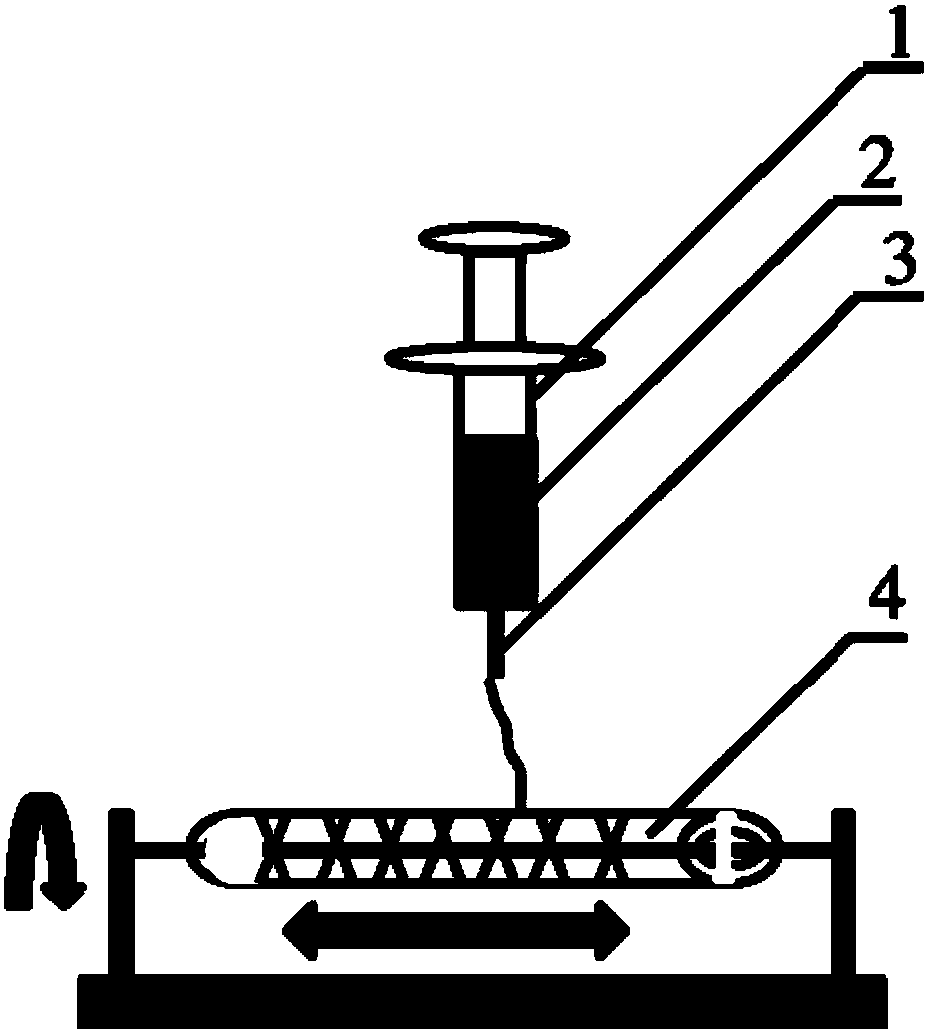
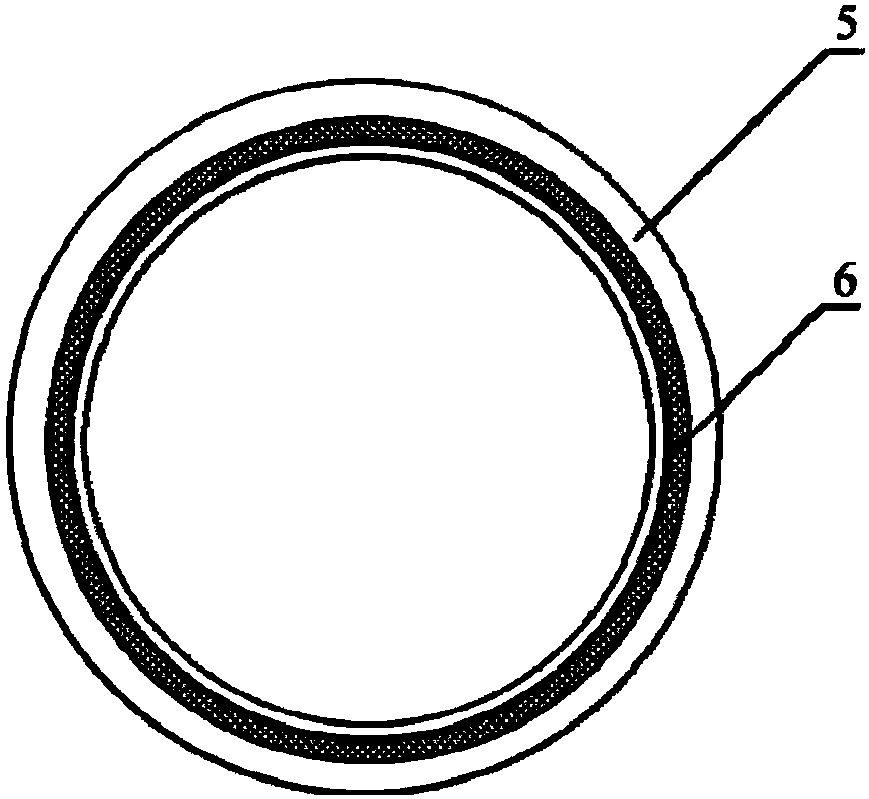
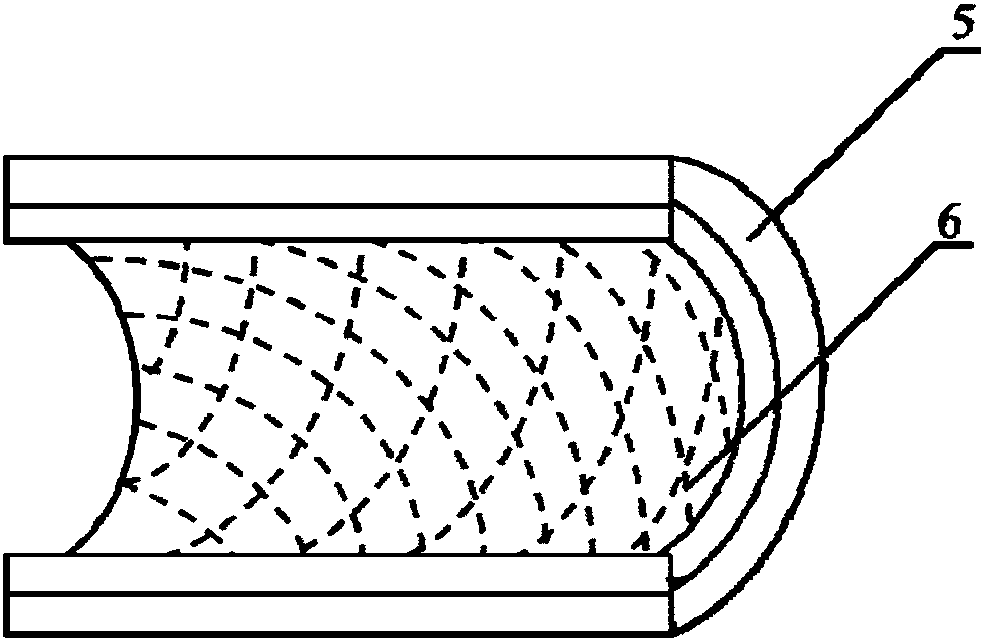
[0033] 1. Prepare the melt spinning fiber skeleton with polycaprolactone (PCL) as spinning fiber material; Take 5.0g by weighing, the number average molecular weight is 80000PCL, place in the closed rust-free steel syringe that thermal melter wraps, in Heat at 200°C for 1h. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The speed is set to 300r / min, and the translation speed is set to 10mm / s. The receiving output is 45 times, and the composite mandrel of PCL melt-spun fiber and medical silicone tube with a fiber angle of 45° is prepared. The diameter of the PCL melt-spun fiber is 60 μm; the wall of the fiber skeleton tubular structure is 250 μm. In the ice-water mixture, take it out after 5s and let it dry for later use.
[0034] 2. Preparation of engineered arterial vessels in vivo: After anesthetizing the rat, prepare the skin on the back, cut an o...
Embodiment 2
[0039] Embodiment 2. The difference between embodiment 2 and embodiment 1 is that the preparation method of the melt-spun fiber skeleton is different;
[0040] Weigh 5.0 g of PCL with a number average molecular weight of 80,000, place it in a closed stainless steel syringe wrapped in a fuser, and heat it at 200 °C for 1 h. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The distance between the syringe needle and the receiver is 2mm, the flow rate of the PCL melt is 0.1ml / h, and the rotation of the receiving rod The speed is set to 300r / min, and the translation speed is set to 5mm / s. The receiving output is 200 times, and the composite mandrel of PCL melt-spun fiber and medical silicone tube with a fiber angle of 30° is prepared. The diameter of the spun fiber is 5 μm; the wall thickness of the tubular structure of the prepared PCL fiber skeleton is 250...
Embodiment 3
[0041] Embodiment 3. The difference between embodiment 3 and embodiment 1 is only different in the preparation method of the melt spinning fiber skeleton;
[0042] Weigh 5.0 g of PGA with a number average molecular weight of 100,000, place it in a closed stainless steel syringe wrapped in a fuser, and heat at 300°C for 1 hour. Put a medical silicone tube with an outer diameter of 2mm and an inner diameter of 1mm on a stainless steel rod with a diameter of 1mm and connect it to the rotating motor. The speed is set to 12r / min, and the translation speed is set to 50mm / s. The receiving output is 60 times, and the composite mandrel of PGA melt-spun fiber and medical silicone tube with a fiber angle of 130° is prepared. The diameter of the spun fiber is 50 μm; the wall thickness of the fiber skeleton tubular structure is 300 μm; after the spinning is completed, the silicone tube is withdrawn from the stainless steel rod.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com