Synthesis method and product of non-steroidal anti-inflammatory drug-chitosan
A technology of non-steroidal anti-inflammatory drugs and synthetic methods, which is applied in the field of drug synthesis, can solve problems such as complicated methods, high cost, and inconvenient expansion of production, and achieve the effects of reducing costs, simplifying synthetic processes, and facilitating industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
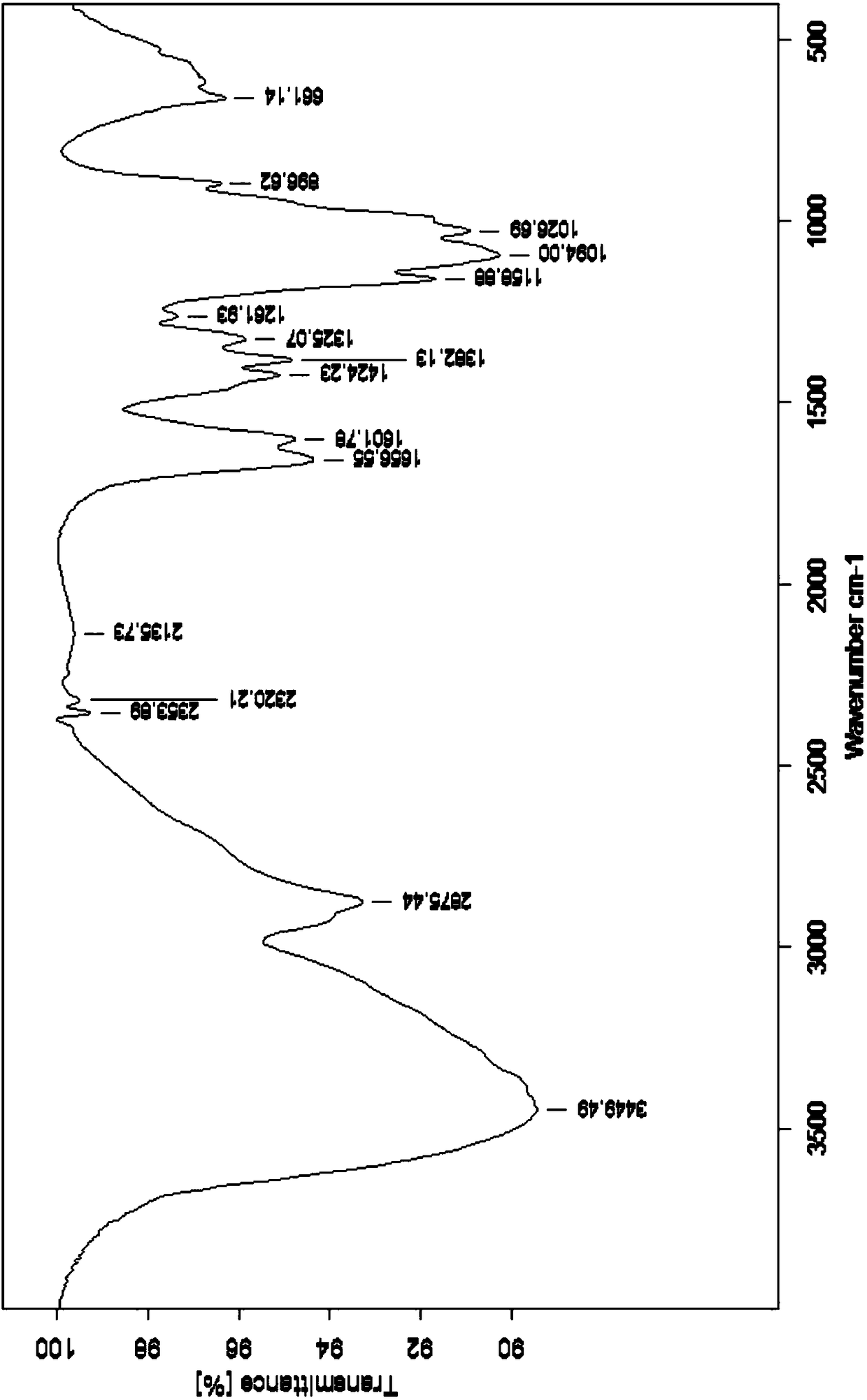
Image
Examples
preparation example Construction
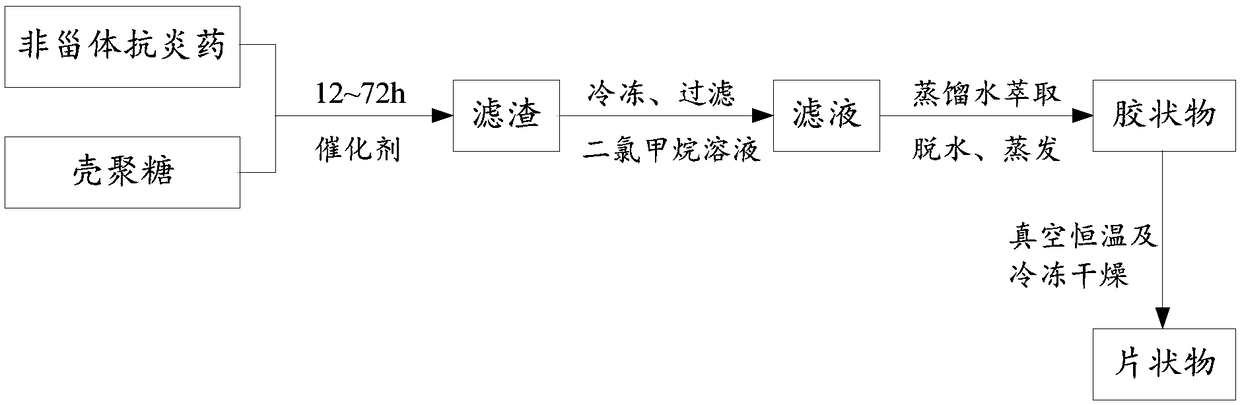
[0038] see figure 1 , the invention provides a kind of synthetic method of NSAID-chitosan, comprising:
[0039] 1. Add 5-50ml of dichloromethane and 0.5-5.0g of chitosan into the reaction vessel and shake well;
[0040] Wherein, the dichloromethane is anhydrous dichloromethane, and the present invention selects dichloromethane, because chitosan and most non-steroidal anti-inflammatory drugs are easier to dissolve therein, and are volatile and easy to remove.
[0041] The chitosan is dried at 55-90° C. for 1-5 hours. Preferably, the chitosan is dried at 60-80° C., and the drying time is 1-3 hours. More preferably, the chitosan is dried at 65-75°C for 1-2 hours. The chitosan is specially dried to remove moisture to the greatest extent without destroying its structure, which is beneficial for chemical reactions to occur under anhydrous conditions.
[0042] 2. Add 0.6-6.0g non-steroidal anti-inflammatory drugs and 1.0-10.0g activated 3A molecular sieve, mix well and stir to di...
Embodiment 1
[0071] 1. Add 5ml of anhydrous dichloromethane and 0.5g of chitosan to the reaction vessel, shake well, wherein the chitosan is dried at 55°C for 1 hour;
[0072] 2. Add 0.6g of non-steroidal anti-inflammatory drugs and 1.0g of activated 3A molecular sieves, mix well and stir to dissolve. Among them, the non-steroidal anti-inflammatory drugs are dried at 55°C for 1 hour; the activated 3A molecular sieves are dried at 90°C for 5 hours;
[0073] 3. Put the reaction vessel under an ice-water bath, add 0.3 g of N,N1-dicyclohexylcarbodiimide and 0.01 g of 4-dimethylaminopyridine, continue to stir, slowly warm up to room temperature, and react for 12 hours;
[0074] 4. Filter the reaction solution to remove molecular sieves and insoluble matter, wash the filter residue with 1ml of dichloromethane, freeze the filtrate overnight, then filter and wash, repeat several times until no precipitation occurs;
[0075] 5. Repeatedly washing and extracting the dichloromethane solution with dist...
Embodiment 2
[0077] 1. Add 10ml of anhydrous dichloromethane and 1.0g of chitosan to the reaction vessel, shake well, wherein the chitosan is dried at 60°C for 2 hours;
[0078] 2. Add 1.0 g of non-steroidal anti-inflammatory drugs and 2.0 g of activated 3A molecular sieves, mix well and stir to dissolve, wherein, the non-steroidal anti-inflammatory drugs are dried at 65°C for 2 hours; the activated 3A molecular sieves are dried at 100°C for 10 hours;
[0079] 3. Put the reaction vessel under an ice-water bath, add 0.5 g of N,N1-dicyclohexylcarbodiimide and 0.05 g of 4-dimethylaminopyridine, continue to stir, slowly warm up to room temperature, and react for 20 hours;
[0080] 4. Filter the reaction solution to remove molecular sieves and insoluble matter, wash the filter residue with 2ml of dichloromethane, freeze the filtrate overnight, then filter and wash, repeat several times until no precipitation occurs;
[0081] 5. Repeatedly washing and extracting the dichloromethane solution wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com