Synthetic method for terbutaline sulfate
A technology of terbutaline sulfate and synthetic method, which is applied in the field of medicine, can solve the problems of large environmental pollution, long steps, and low yield, and achieve the effects of small environmental pollution, short steps, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
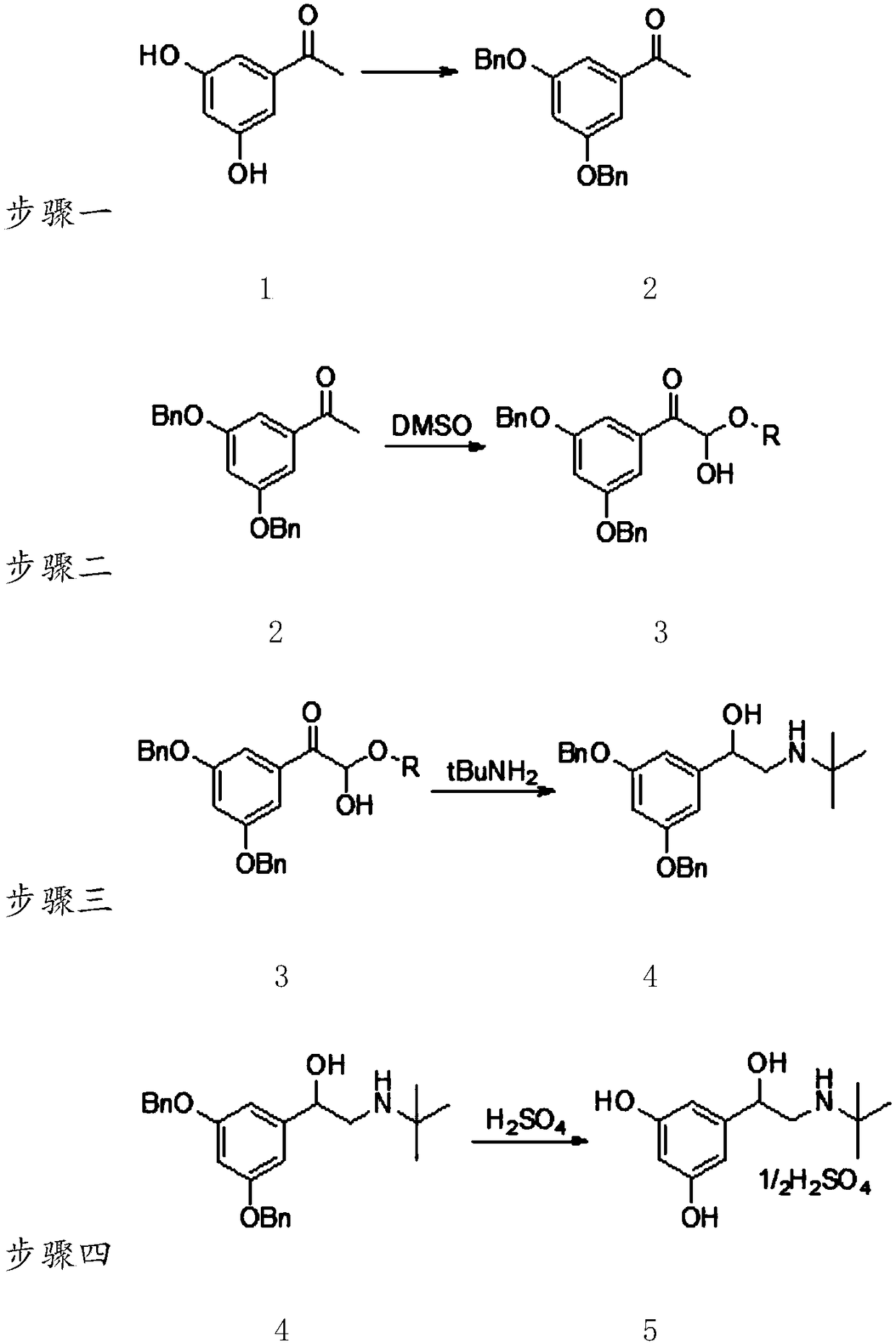
[0041] A kind of synthetic method of terbutaline sulfate, see appendix figure 1 The shown reaction step diagram specifically includes:
[0042] (1) Synthesis of 3,5-dibenzyloxyacetophenone
[0043] 20.0g (0.13mol) of 3,5-dihydroxyacetophenone was dissolved in 150g N,N-dimethylformamide (DMF), and 90.5g potassium carbonate (0.65mol) was added while stirring slowly, and 77.8 mL benzyl chloride (0.68mol) was reacted at 80-90°C for 3-4 hours. Then cool down and filter to remove the solid, rinse the filter cake with a small amount of N,N-dimethylformamide solvent, pour the filtrate into ice water, and precipitate a white solid, wash and dry to obtain a white solid, namely 3,5-dibenzyloxy Acetophenone 44.8g (0.11mol), the molar yield is 85%.
[0044] 1 H NMR (300MHz, CDCl 3 ): δ=2.55(3H,d), 5.07(4H,t), 6.81(1H,m), 7.20(2H,m), 7.38(6H,d), 7.47(4H,d)
[0045] (2) Synthesis of 1-(3,5-bis(benzyloxy)phenyl)-2-ethoxy-2-hydroxyethanone
[0046] Dissolve 50.0 g (0.15 mol) of 3,5-dibenz...
Embodiment 2
[0055] A kind of synthetic method of terbutaline sulfate, see appendix figure 1 The shown reaction step diagram specifically includes:
[0056] (1) Synthesis of 3,5-dibenzyloxyacetophenone
[0057] 100.0g (0.66mol) of 3,5-dihydroxyacetophenone was dissolved in 700g dimethyl sulfoxide (DMSO), and 212.5g triethylamine (2.1mol) was added under stirring, and 282.2g benzyl bromide (1.65 mol) at 70-80°C for 3-4 hours. Then cool down and filter to remove the solid, rinse the filter cake with a small amount of solvent, pour the filtrate into ice water, and precipitate a white solid, wash and dry to obtain a white solid, namely 189.5g (0.57mol) of 3,5-dibenzyloxyacetophenone , 87% molar yield.
[0058] 1 H NMR (300MHz, CDCl 3 ): δ=2.55(3H,d), 5.07(4H,t), 6.81(1H,m), 7.20(2H,m), 7.38(6H,d), 7.47(4H,d)
[0059] (2) Synthesis of 1-(3,5-bis(benzyloxy)phenyl)-2-ethoxy-2-hydroxyethanone
[0060] Dissolve 80g (0.24mol) of 3,5-dibenzyloxyacetophenone in 450g DMSO, add 76.1g refined iodi...
Embodiment 3
[0069] A kind of synthetic method of terbutaline sulfate, see appendix figure 1 The shown reaction step diagram specifically includes:
[0070] (1) Synthesis of 3,5-dibenzyloxyacetophenone
[0071] 20.0g (0.13mol) of 3,5-dihydroxyacetophenone was dissolved in 160g of N,N-dimethylacetamide, 55g of sodium carbonate (0.52mol) was added while stirring slowly, and 38.9mL of benzyl chloride ( 0.34mol) at 60-70°C for 3-4 hours. Then cool down and filter to remove the solid, rinse the filter cake with a small amount of solvent, pour the filtrate into ice water, and precipitate a white solid, wash and dry to obtain a white solid, namely 40.7g (0.1mol) of 3,5-dibenzyloxyacetophenone , the molar yield was 82%.
[0072] (2) Synthesis of 1-(3,5-bis(benzyloxy)phenyl)-2-ethoxy-2-hydroxyethanone
[0073] Dissolve 60.0g (0.18mol) of 3,5-dibenzyloxyacetophenone in 400g DMSO, add 180ml of 50% hydroiodic acid (0.9mol) at 40-50°C, and then raise the temperature to 70-80°C for reaction After 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com