Method for efficient in-vitro amplification of peripheral blood NK cells
An in vitro expansion and NK cell technology, applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., can solve the problems of limited NK cell application, low NK cell content, low distribution frequency, etc., to reduce cellular immunity. The effect of originality, ensuring amplification multiple, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
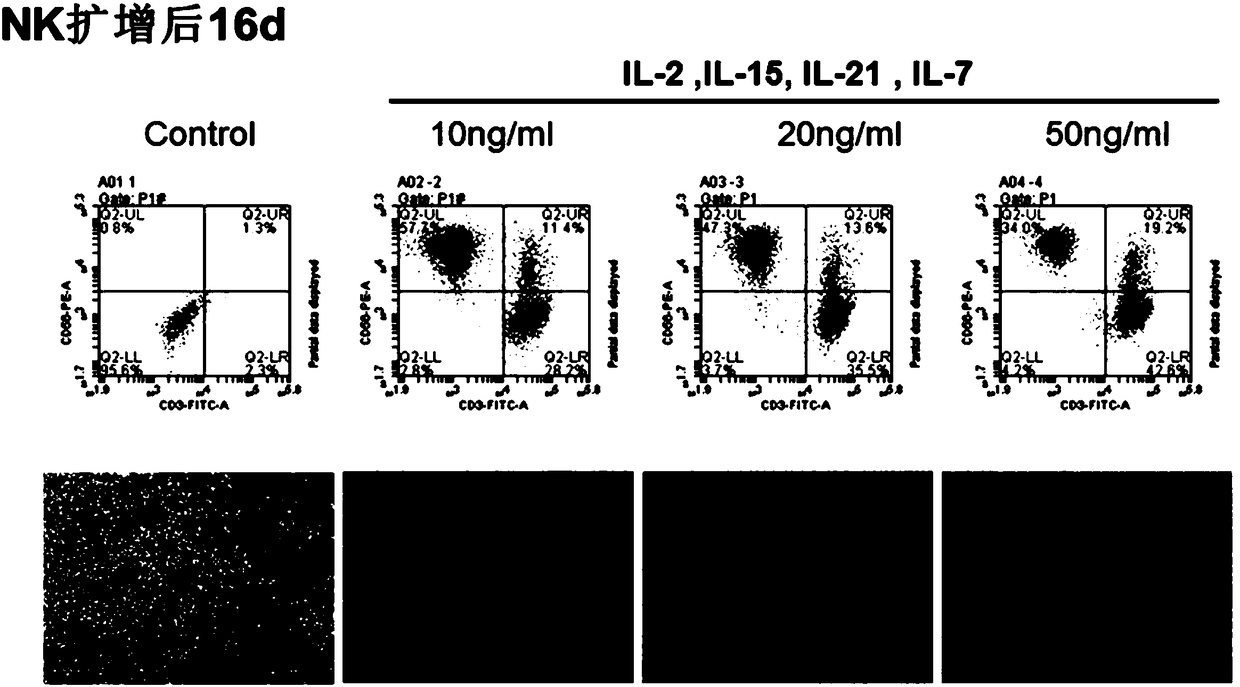
Image
Examples
Embodiment 1
[0036] A method for efficiently expanding peripheral blood NK cells in vitro, comprising the following steps:
[0037] 1) Under sterile conditions, take 10 mL of whole blood and anticoagulate it with heparin sodium to obtain anticoagulated whole blood, then dilute the anticoagulated whole blood with 10 mL of PBS, wherein the volume ratio of the whole blood to heparin sodium is 5000:10, wherein The concentration of heparin sodium is 6250U / mL.
[0038] 2) Take another 10 mL of lymphocyte separation medium, slowly spread the diluted anticoagulated whole blood on the surface of the lymphocyte separation medium, and keep the interface between the two liquid surfaces clear.
[0039] 3) Centrifuge the liquid obtained in step 2) at room temperature (use a horizontal rotor to centrifuge at a rate of 2000rpm for 15min), absorb a thin and dense buffy coat layer between the plasma layer and the separation liquid layer, and place the buffy coat layer on the In another centrifuge tube, blo...
Embodiment 2
[0043] A method for efficiently expanding peripheral blood NK cells in vitro, comprising the following steps:
[0044] 1) Under sterile conditions, take 10 mL of whole blood and anticoagulate it with heparin sodium to obtain anticoagulated whole blood, and then dilute the anticoagulated whole blood with 10 mL of PBS, wherein the volume ratio of the whole blood to heparin sodium is 5000:10, wherein The concentration of heparin sodium is 6250U / mL.
[0045] 2) Take another 10 mL of lymphocyte separation medium, slowly spread the diluted anticoagulated whole blood on the surface of the lymphocyte separation medium, and keep the interface between the two liquid surfaces clear.
[0046] 3) Centrifuge the liquid obtained in step 2) at room temperature (centrifuge at a speed of 2500 rpm for 13 min with a horizontal rotor), absorb a thin and dense buffy coat layer between the plasma layer and the separation liquid layer, and place the buffy coat layer on In another centrifuge tube, bl...
Embodiment 3
[0050] A method for efficiently expanding peripheral blood NK cells in vitro, comprising the following steps:
[0051] 1) Under sterile conditions, take 10 mL of whole blood and anticoagulate it with heparin sodium to obtain anticoagulated whole blood, and then dilute the anticoagulated whole blood with 10 mL of PBS, wherein the volume ratio of the whole blood to heparin sodium is 5000:10, wherein The concentration of heparin sodium is 6250U / mL.
[0052] 2) Take another 10 mL of lymphocyte separation medium, slowly spread the diluted anticoagulated whole blood on the surface of the lymphocyte separation medium, and keep the interface between the two liquid surfaces clear.
[0053] 3) Centrifuge the liquid obtained in step 2) at room temperature (use a horizontal rotor to centrifuge at a rate of 1500rpm for 17min), absorb a thin and dense buffy coat layer between the plasma layer and the separation liquid layer, and place the buffy coat layer on In another centrifuge tube, blo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com