Application of a mononuclear nickel complex as a catalyst for electrocatalytic hydrogen production and photocatalytic degradation of organic dyes
A nickel complex, electrocatalysis technology, applied in the direction of organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of less research on mononuclear nickel complex photocatalysis, To achieve the effect of simple synthesis method, high synthesis yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
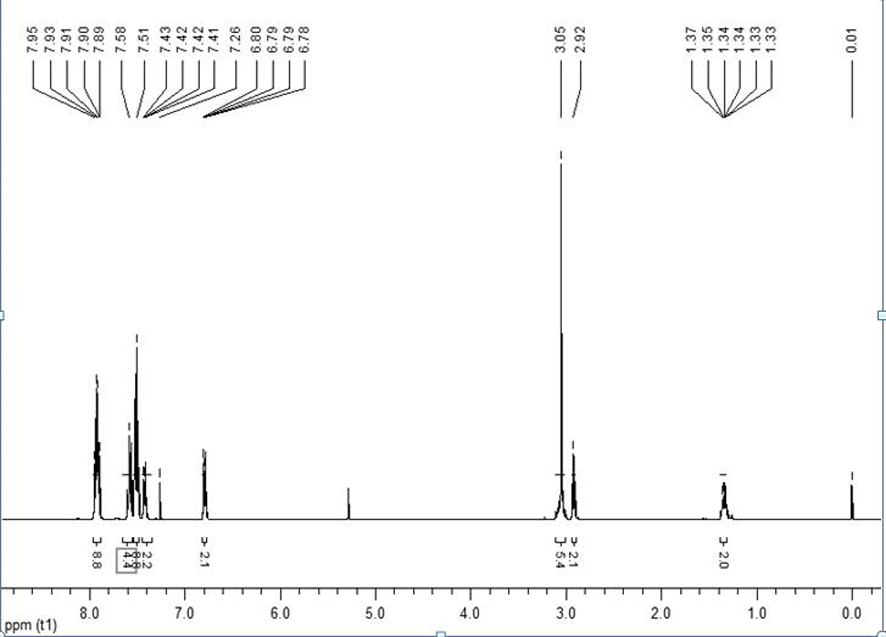
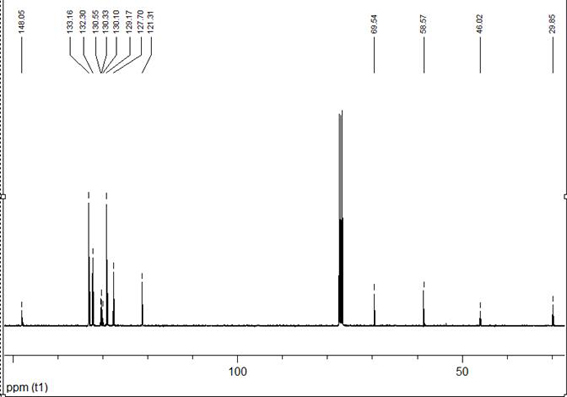
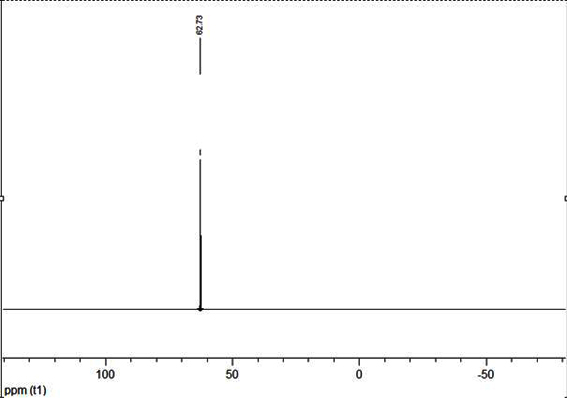
[0033] A kind of mononuclear nickel complex containing N-substituted diphosphine ammonia and 1,2-benzenedithiophenate ligand, the chemical formula of the mononuclear nickel complex is (CH 3 O(CH 2 ) 3 N(PPh 2 ) 2 )Ni(bdt), its synthetic reaction is as follows:
[0034]
[0035] The synthetic method of the mononuclear nickel complex containing N-substituted bisphosphine ammonia and 1,2-benzenedithiophenate ligand is as follows:
[0036] In a round bottom flask equipped with a magnetic stirrer bar, 0.0711g H 2 bdt (0.5mmol), 0.2925g (CH 3 O(CH 2 ) 3 N(PPh 2 ) 2 )NiCl 2 (0.5mmol) and 20mL of dichloromethane were mixed and stirred at room temperature for 3 hours to obtain a purple solution. The solvent was removed by a rotary evaporator under reduced pressure, dissolved in an appropriate amount of dichloromethane, and separated by thin-layer chromatography using dichloromethane as a developing solvent. The red band was collected, and 0.2922 g was obtained after eluti...
Embodiment 2
[0038] A kind of mononuclear nickel complex containing N-substituted diphosphine ammonia and 1,2-benzenedithiophenate ligand, the chemical formula of the mononuclear nickel complex is (CH 3 S(CH 2 ) 3 N(PPh 2 ) 2 )Ni(bdt), its chemical structure is as follows:
[0039]
[0040] The synthetic method of the mononuclear nickel complex containing N-substituted bisphosphine ammonia and 1,2-benzenedithiophenate ligand is as follows:
[0041] In a round bottom flask equipped with a magnetic stirrer bar, 0.0711g H 2 bdt (0.5mmol), 0.3005g (CH 3 S(CH 2 ) 3 N(PPh 2 ) 2 )NiCl 2 (0.5mmol) and 20mL of dichloromethane were mixed and stirred at room temperature for 3 hours to obtain a purple solution. The solvent was removed by rotary evaporator under reduced pressure, dissolved with an appropriate amount of dichloromethane, and a 2:1 mixture of dichloromethane and petroleum ether was used as a developing solvent for thin-layer chromatography. The red band was collected and 0.2...
Embodiment 3
[0043] A kind of mononuclear nickel complex containing N-substituted diphosphine ammonia and 1,2-benzenedithiophenate ligand, the chemical formula of the mononuclear nickel complex is (CH 3 CH(Ph)N(PPh 2 ) 2 )Ni(bdt), its chemical structure is as follows:
[0044]
[0045] The synthetic method of the mononuclear nickel complex containing N-substituted bisphosphine ammonia and 1,2-benzenedithiophenate ligand is as follows:
[0046] In a round bottom flask equipped with a magnetic stirrer bar, 0.0711g H 2 bdt (0.5mmol), 0.3090g (CH 3 CH(Ph)N(PPh 2 ) 2 )NiCl 2 (0.5mmol) and 20mL of chloroform were mixed, stirred and reacted at room temperature for 2 hours to obtain a purple solution. The solvent was removed by rotary evaporator under reduced pressure, dissolved with an appropriate amount of chloroform, and a 3:1 mixture of dichloromethane and petroleum ether was used as a developing solvent for thin-layer chromatography. The red band was collected and 0.2606g was obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com