Subcutaneous medicine controllable release biological needle and preparation method thereof
A drug and biological technology, applied in the direction of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of time-consuming and labor-intensive, and achieve the effect of low price, small size, and controlled drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
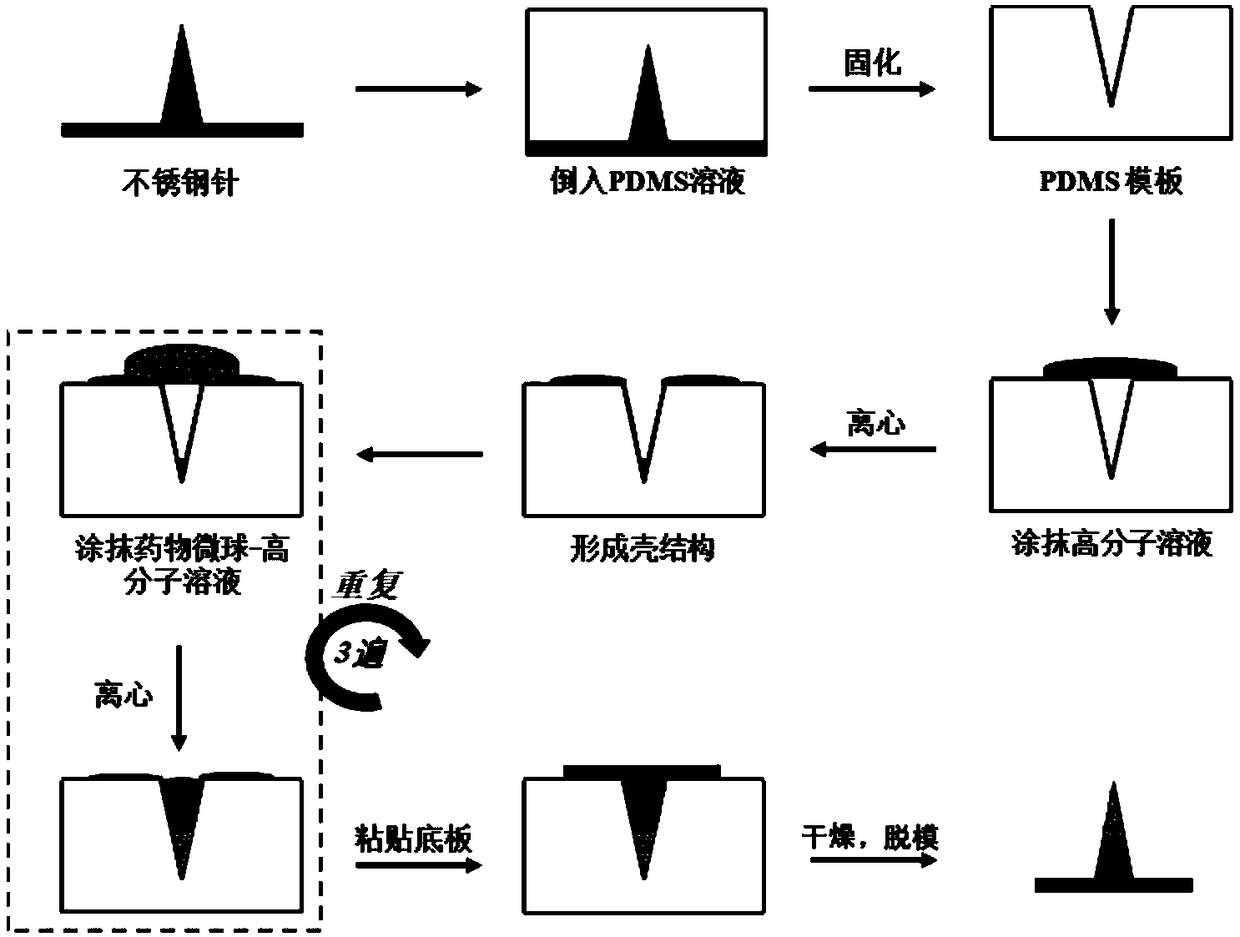
[0030] Example 1 The preparation of a biological needle with a height of 3mm and a bottom diameter of 1.2mm
[0031] Proceed as follows:
[0032] (1) Preparation of biological needle template: Prepare a stainless steel needle with a tip by electric grinding and polishing, and place the steel needle vertically in a horizontal container so that the steel needle is 3 mm higher than the bottom of the container, and the diameter of the plane in contact with the bottom of the container is 1.2mm. Weigh 100g of polydimethylsiloxane and 10g of curing agent to mix, stir evenly and remove the air bubbles under vacuum conditions, then pour the liquid polydimethylsiloxane containing curing agent with the removed air bubbles on a stainless steel needle In the container, place it in an oven at 60°C for 6 hours and then release the mold to obtain a polydimethylsiloxane template with a depth of 3 mm and a bottom diameter of 1.2 mm, which is packaged for use;
[0033] (2) Preparation of solut...
Embodiment 2
[0035] Embodiment 2 in vitro skin penetration test
[0036] Proceed as follows:
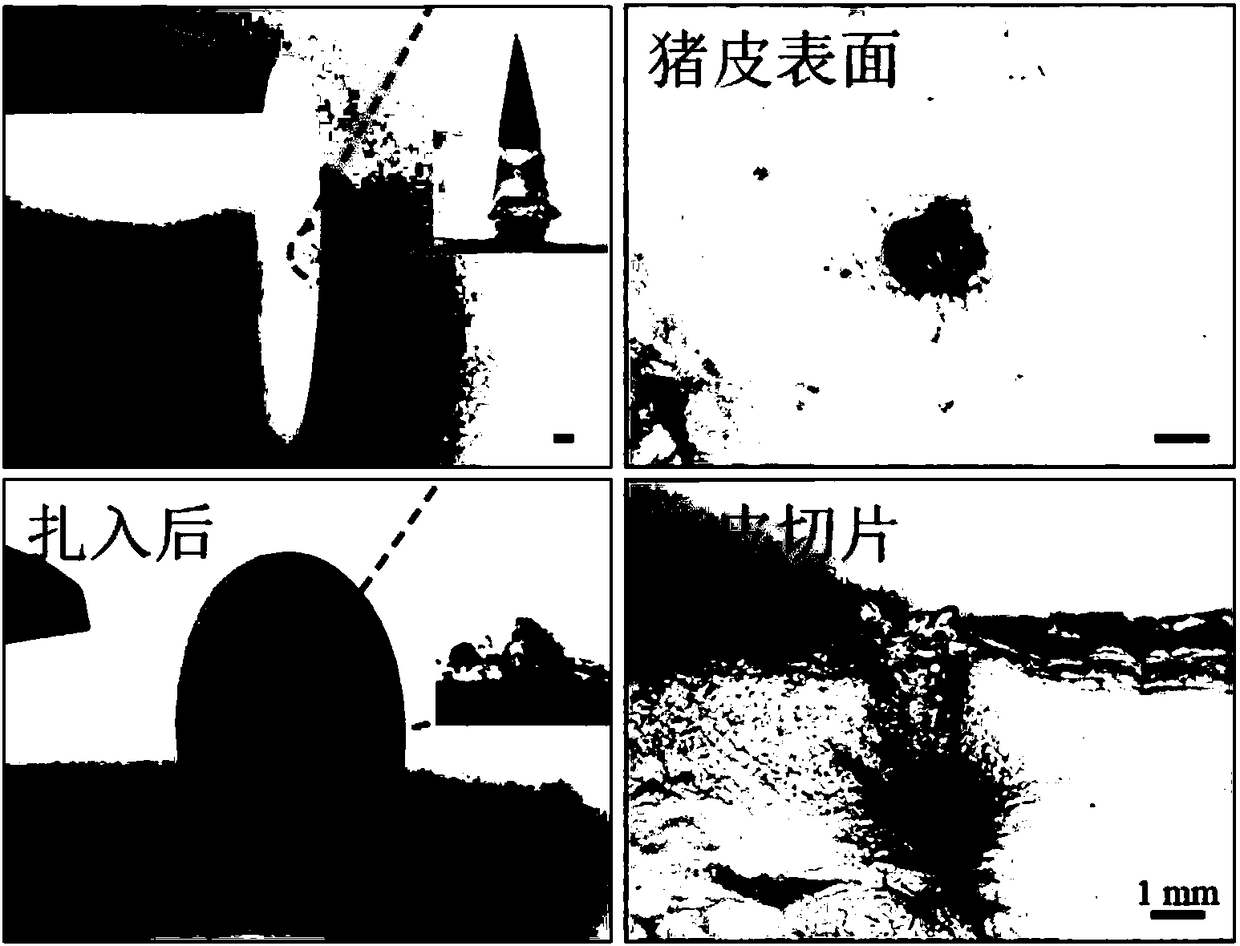
[0037] Disinfect the flat fresh dehaired pigskin, take the dried biological needle and install it on a self-made hand-held drag-and-drop device, and align the needle point of the biological needle with the surface of the pigskin and insert it vertically. Use an optical microscope to observe the biological needles and pigskin before and after piercing the pigskin, and slice the pigskin along the pinholes to observe the tissue sections of the pigskin.
[0038] It can be observed under an optical microscope that the height of the biological needle installed on the drag-and-drop device is 3mm and the bottom diameter is 1.2mm before being pierced into the pigskin. After piercing into the pigskin, only a little bit of polymer remained on the dropper with the bioneedle installed, indicating that the entire bioneedle was completely pierced into the pigskin. In addition, there is a clear pinhole on the su...
Embodiment 3
[0039] Embodiment 3 in vitro subcutaneous dissolution test
[0040] Proceed as follows:
[0041] At room temperature, take a number of completely dry biological needles and install them on the automatic dynamometer in 5 groups. Use a constant force of 10N to pierce the biological needles into fresh hair-free pigskin and stay for 2, 5, 10, 20 and 30 minutes respectively. The array was removed, and the shape changes of the biological needles after staying subcutaneously for different periods of time were observed under a microscope. The results showed that 2 minutes after the biological needle was inserted into the skin, the tip of the needle began to dissolve, exposing the encapsulated drug microspheres. After 10 minutes of subcutaneous insertion, it can be seen that the needle tip of the bioneedle is completely dissolved in the subcutaneous tissue, and most of the drug microspheres are released into the subcutaneous tissue. After 20 minutes, all the drug microspheres are rel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com