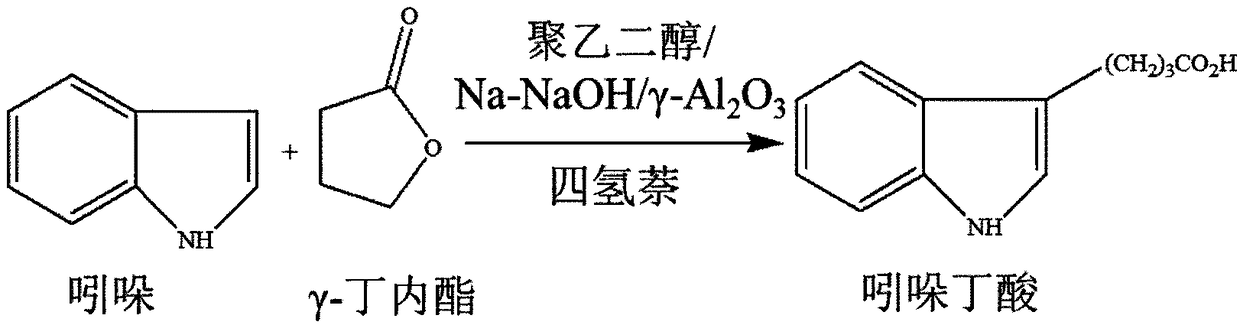
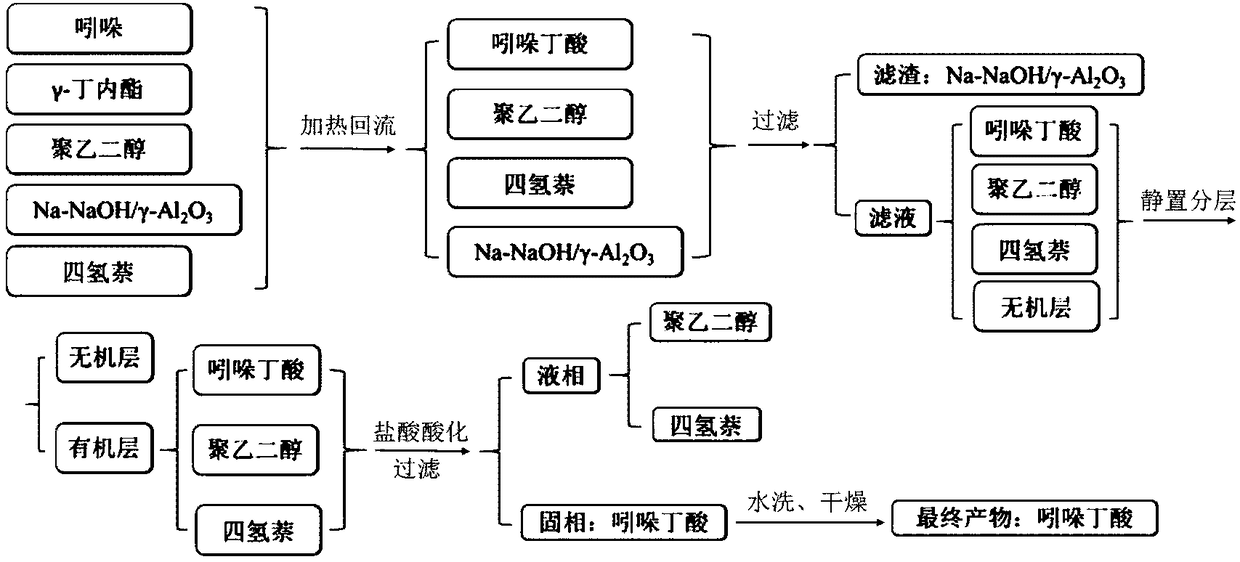
3-indolebutyric acid preparation method
A technology for indole butyric acid and indole, which is applied in the field of preparing 3-indole butyric acid, can solve the problems of harsh conditions, many synthesis steps, low yield and the like, and achieves the advantages of good effect, simple preparation method and easy recycling. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Solid super base Na-NaOH / γ-Al 2 o 3 Preparation: Add 100g γ-Al to the reactor 2 o 3 , under the conditions of vigorous stirring and nitrogen purging, slowly heat up to 100° C., and keep for 1 hour to remove water adsorbed on the surface of the carrier. The temperature was raised to 200°C, and 30 g of NaOH was added under the condition of vigorous stirring and nitrogen purging, and kept for 1 h. After that, 10 g of sodium metal was added quickly, and stirring was continued for 1 h. Cool to get solid super base Na-NaOH / γ-Al 2 o 3 catalyst.
[0027] Indole, γ-butyrolactone, tetralin, polyethylene glycol and Na-NaOH / γ-Al 2 o 3 Join in the reactor, wherein the loading amount of indole, gamma-butyrolactone and tetrahydronaphthalene is respectively 11.7g, 10.3g and 35.1g, the loading amount of polyethylene glycol is 16% of indole, Na -NaOH / γ-Al 2 o 3 The loading amount is 2% of indole. Start the stirring function of the heating agitator and stir at room temperature...
Embodiment 2
[0030] Solid super base Na-NaOH / γ-Al 2 o 3 Preparation: Add 100g γ-Al to the reactor 2 o 3 , under the conditions of vigorous stirring and nitrogen purging, slowly heat up to 200° C., and keep for 5 hours to remove water adsorbed on the surface of the carrier. The temperature was raised to 400°C, and 30 g of NaOH was added under strong stirring and nitrogen purging, and kept for 3 h. After that, 10 g of sodium metal was added quickly, and stirring was continued for 3 h. Cool to get solid super base Na-NaOH / γ-Al 2 o 3 catalyst.
[0031] Indole, γ-butyrolactone, tetralin, polyethylene glycol and Na-NaOH / γ-Al 2 o 3 Join in the reactor, wherein the loading amount of indole, gamma-butyrolactone and tetrahydronaphthalene is respectively 11.7g, 11.2g and 58.5g, the loading amount of polyethylene glycol is 17% of indole, Na -NaOH / γ-Al 2 o 3 The loading amount is 5% of indole. Start the stirring function of the heating stirrer and stir at room temperature for 30 minutes to ...
Embodiment 3
[0034] Solid super base K-KOH / γ-Al 2 o 3Preparation: Add 100g γ-Al to the reactor 2 o 3 , under the conditions of vigorous stirring and nitrogen purging, slowly heat up to 100° C., and keep for 1 hour to remove water adsorbed on the surface of the carrier. The temperature was raised to 200°C, and 30 g of KOH was added under vigorous stirring and nitrogen purging, and kept for 1 h. Afterwards, 10 g of metal K was added quickly and stirring was continued for 1 h. Cool to get solid super base K-KOH / γ-Al 2 o 3 catalyst.
[0035] Indole, γ-butyrolactone, tetralin, polyethylene glycol and K-KOH / γ-Al 2 o 3 Join in the reactor, wherein the loading amount of indole, gamma-butyrolactone and tetrahydronaphthalene is respectively 11.7g, 10.3g and 35.1g, the loading amount of polyethylene glycol is 16% of indole, K -KOH / γ-Al 2 o 3 The loading amount is 2% of indole. Start the stirring function of the heating agitator and stir at room temperature for 20 minutes to mix the five c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com