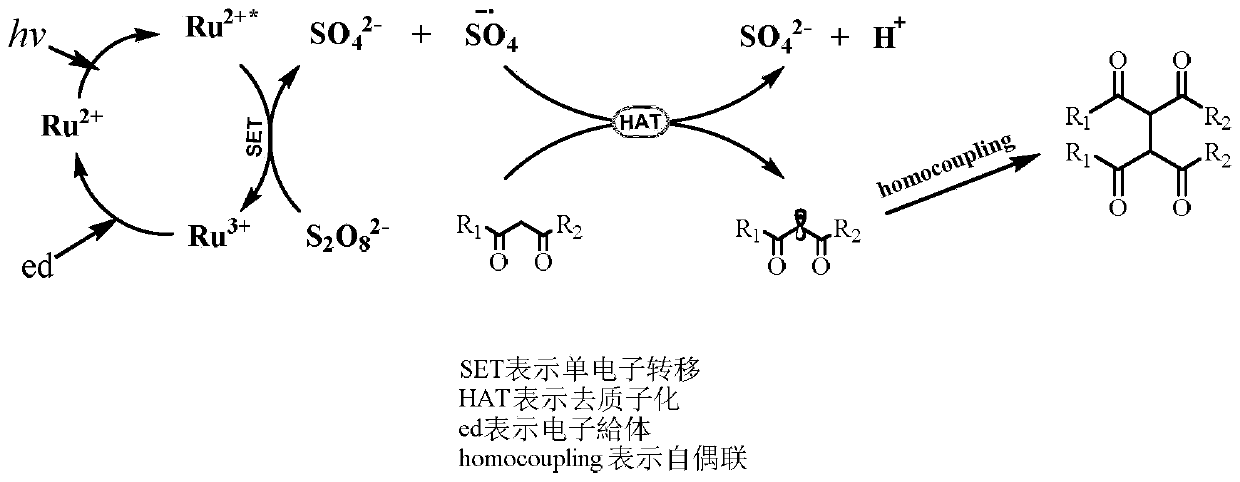
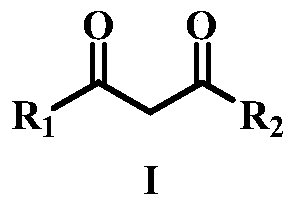
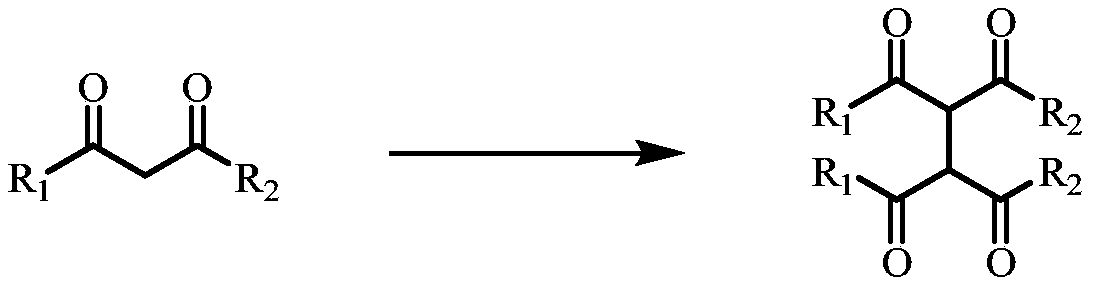
A method for the dehydrogenation self-coupling of 1,3-dicarbonyl compounds catalyzed by visible light
A dicarbonyl and compound technology, applied in the field of organic catalysis, can solve the problems of cumbersome preparation work, easy occurrence of by-products, large yield loss, etc., and achieve the effects of improving energy utilization, reducing solvent polarity, and improving efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Weigh terpyridyl ruthenium(II) chloride hexahydrate (0.006mmol, 2% equiv) and potassium persulfate (0.45mmol, 1.5equiv) into the reactor, add ethyl benzoyl acetate (0.3mmol, 1equiv) , 1,4-dioxane (0.15mmol, 0.5equiv), mixed with solvent acetonitrile / water (V / V=1mL / 1mL). Put the reaction bottle into an oil bath at 25°C, and react for 24 hours under the irradiation of a 26W LED lamp. After the reaction, separation and purification were carried out to obtain product 1 (the reaction yield was 88%).
Embodiment 2
[0041] Weigh terpyridyl ruthenium (II) chloride hexahydrate (0.006mmol, 2% equiv) and potassium persulfate (0.45mmol, 1.5equiv) into the reactor, add ethyl p-fluorobenzoyl acetate (0.3mmol, 1equiv), 1,4-dioxane (0.15mmol, 0.5equiv), with the solvent acetonitrile / water (V / V=1mL / 1mL). Put the reaction bottle into an oil bath at 25°C, and react for 24 hours under the irradiation of a 26W LED lamp. After the reaction, separation and purification were carried out to obtain the product 2 (the reaction yield was 82%).
Embodiment 3
[0043] Weigh terpyridyl ruthenium (II) chloride hexahydrate (0.006mmol, 2% equiv) and potassium persulfate (0.45mmol, 1.5equiv) into the reactor, add ethyl p-chlorobenzoyl acetate (0.3mmol, 1equiv), 1,4-dioxane (0.15mmol, 0.5equiv), with the solvent acetonitrile / water (V / V=1mL / 1mL). Put the reaction bottle into an oil bath at 25°C, and react for 24 hours under the irradiation of a 26W LED lamp. After the reaction, separation and purification were carried out to obtain the product 3 (the reaction yield was 78%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com