RT/PR dual-target HIV inhibitor, preparation method and applications thereof
A technology of inhibitors and double-substituted amine groups, applied in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve problems such as large doses, cross-resistance, and complex drug interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
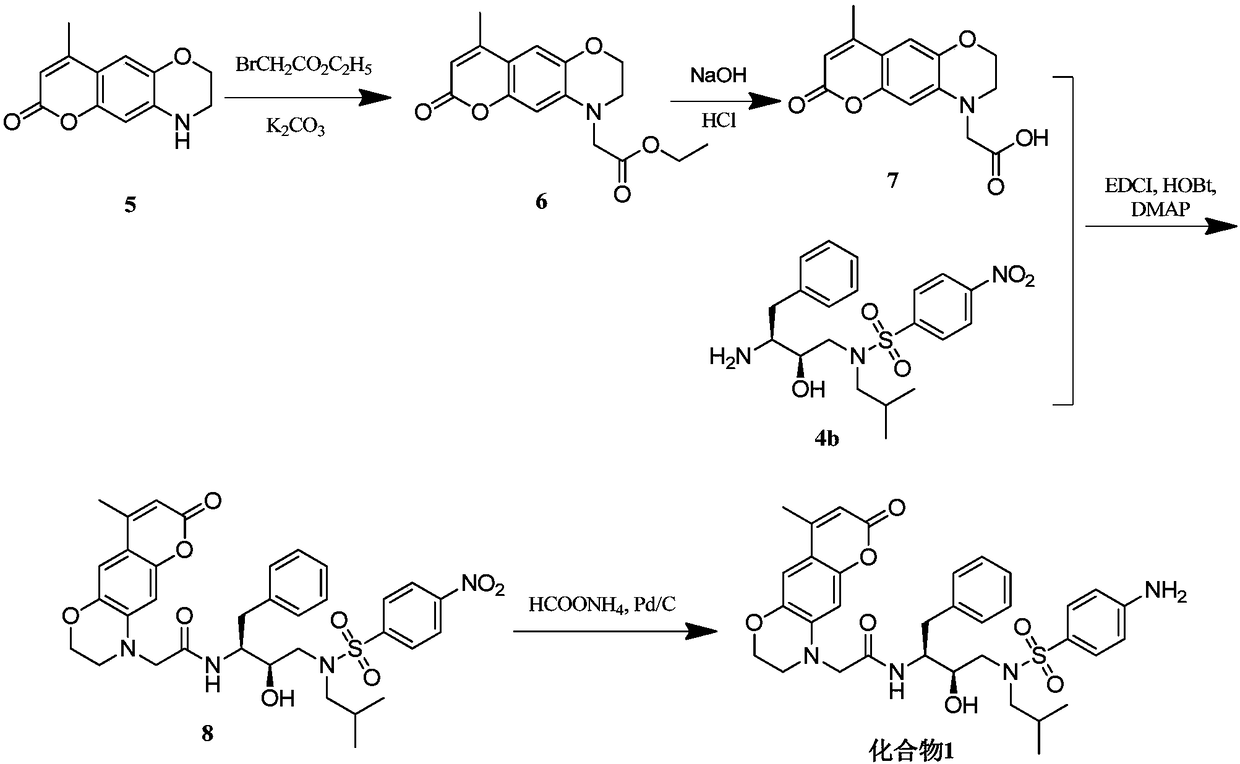
[0058] Example 1, 2-(8-methyl-3,4-dihydro-1,5-dioxo-6-oxo-anthracene-4-amino)-N-((2S,3R)-3-hydroxy -Synthesis of 4-(N-isobutyl-4-aminophenylsulfonamido)-1-phenylbutan-2-yl)-acetamide (namely compound 1)
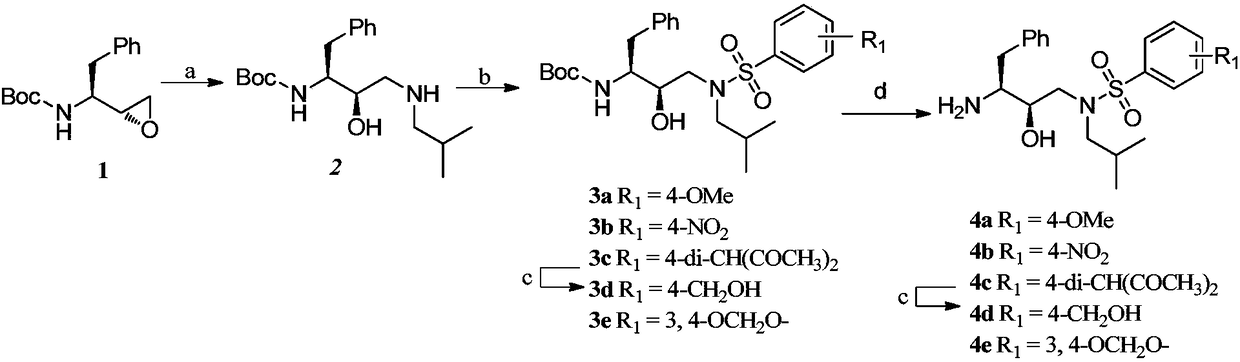
[0059] For the synthetic route of compound 1, see figure 2 , the specific operation is as follows:
[0060] 1) Synthesis of tert-butyl (1S,2R)-1-benzyl-2-hydroxy-3-(isobutylamine)carbamate (intermediate 2)
[0061] (S)-1-((S)-oxirane-2-yl)-2-phenylethylcarbamate tert-butyl ester (1) (Bailingwei Technology Co., Ltd.) (20.0g, 75.94mmol), acetonitrile 80mL and isobutylamine (19.02 mL, 189.46 mmol) were added into a 200 mL eggplant-shaped flask, and the mixture was stirred at 80° C. for 5 hours. After the reaction was completed, the reaction liquid was cooled to room temperature, and concentrated under reduced pressure to remove the solvent. The crude product was recrystallized with ethyl acetate / n-hexane (1:9) to obtain a white target product (21.2 g, 83%), which was interme...
Embodiment 2
[0077] Example 2, 2-(7-hydroxyl-2-oxoquinolin-1(2H)-yl)-N-((2S,3R)-3-hydroxyl-4-(N-isobutyl-4- Synthesis of Aminophenylsulfonamido)-1-Phenylbutan-2-yl)-Acetamide (Compound 12)
[0078] The synthetic route of compound 12 is shown in image 3 , the specific operation is as follows:
[0079] 1) Synthesis of 2-oxo-1,2-dihydroquinolin-7-yl-acetate (intermediate 10)
[0080] Dissolve intermediate 9 (7-hydroxy-2-(1H)-quinolinone) (1.61g, 10mmol) (Beijing Yinuokai Technology Co., Ltd.) in 28ml of acetic anhydride, cool to 0°C, add trifluoroform Indium Sulfonate In(OTf) 3 (0.28g, 0.5mmol), raised to room temperature and stirred for 1 hour. Add ethyl acetate and 10% sodium carbonate aqueous solution, continue to stir and react for 1 hour, separate the organic phase, and wash 3 times with saturated sodium bicarbonate solution, dry over anhydrous magnesium sulfate, concentrate to obtain the target product (i.e. intermediate 10) 1.83g, Yield 90.0%. LC-MS (ESI, M+H + ) m / z 204.7.
...
Embodiment 3
[0090] Example 3, 8-(3-methyl-2-buten-1-yl)-2-oxo-2H-chroman-7-yl-(2S,3R)-3-hydroxyl-4-(N Synthesis of -isobutyl-4-aminophenylsulfonamido)-1-phenylbutan-2-yl)-carbamate (i.e. compound 18)
[0091] The synthetic route of compound 18 is shown in Figure 4 , the specific operation is as follows:
[0092] 1) Synthesis of 7-hydroxy-8-(3-methyl-2-buten-1-yl)-2H-chroman-2-one (intermediate 15)
[0093] Anhydrous aluminum trichloride (1.32g, 10.0mmol) was suspended in 20mL of anhydrous dichloromethane, and 10mL of methyl sulfide was slowly added dropwise with stirring at 0°C. After the dropwise addition, the reaction solution became clear. Osthole ( Figure 4 14, 0.98g, 4.0mmol) (Beijing Yinuokai Technology Co., Ltd.) was dissolved in 10mL of anhydrous dichloromethane, added dropwise to the reaction solution, the dropwise addition was completed within 10min, and the reaction was stirred at room temperature for 24 hours. The reaction was quenched with 30 mL of 1M cold dilute hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com