A kind of recombinant human growth hormone injection and preparation method thereof
A technology of human growth hormone and injection, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., can solve the problems of reduced stability and pain, achieve good stability, reduce pain, and enhance use safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
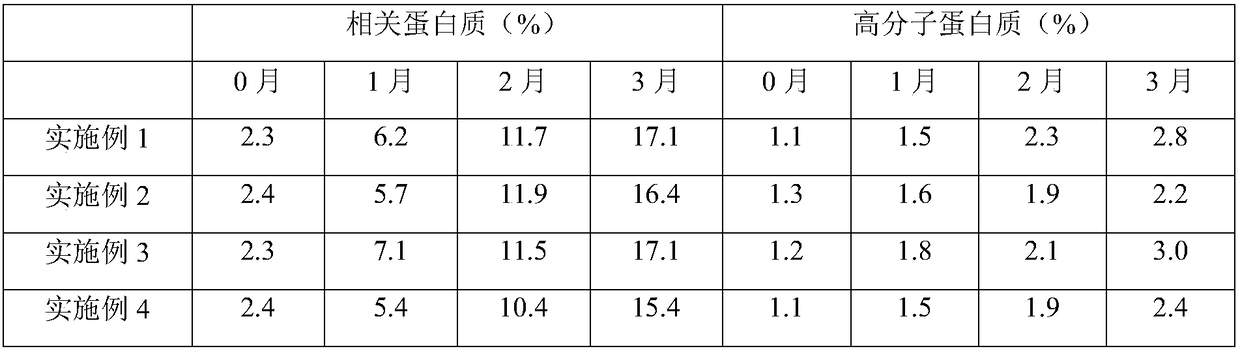
Embodiment 1
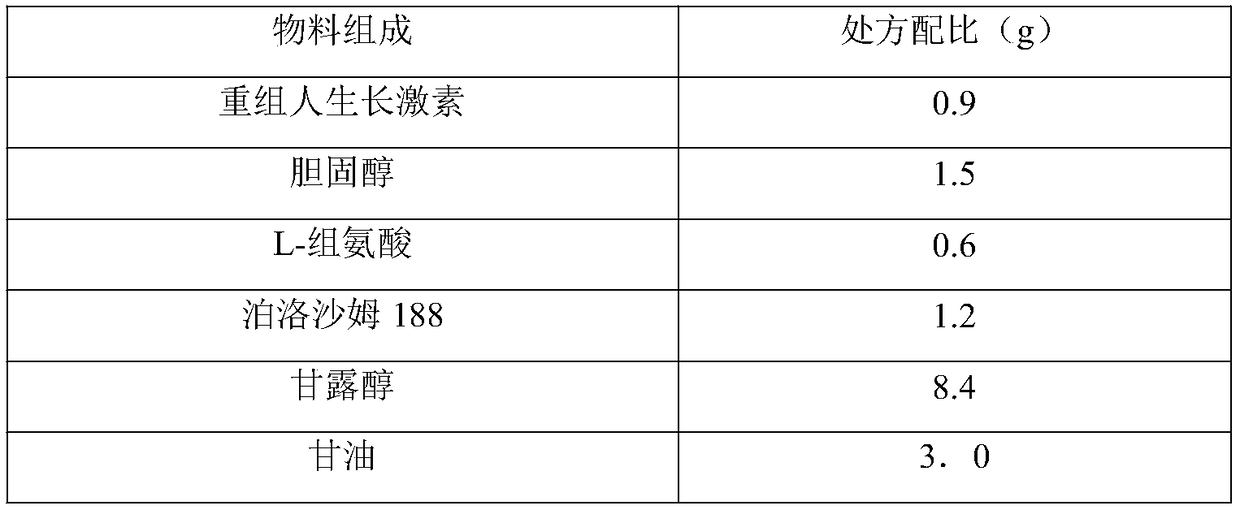
[0013] prescription:
[0014] Material composition
[0015] Preparation method: a. Weigh poloxamer 188 and mannitol, add 30ml of water for injection and stir respectively, fully dissolve and mix, add glycerin diluted with 30ml of water to obtain a stock solution, add recombinant human growth hormone stock solution and cholesterol in the stock solution, set b. In a grade A environment, filter the medicinal solution obtained in step a through a 0.22 μm microporous membrane, and then pack it into a glass sleeve for a pen-type syringe.
Embodiment 2
[0017] prescription:
[0018] Material composition
[0019] Preparation method: a. Weigh L-histidine and mannitol, add 20ml of water for injection and stir respectively, fully dissolve and mix, add glycerin diluted with 40ml of water to obtain a stock solution, add recombinant human growth hormone stock solution and cholesterol to the stock solution, and b. In a grade-A environment, filter the medicinal solution obtained in step a through a 0.22 μm microporous membrane, and pack it separately to obtain the medicinal solution.
Embodiment 3
[0021] prescription:
[0022] Material composition
[0023] Preparation method: a. Weigh L-histidine, poloxamer 188 and mannitol, respectively add 25ml of water for injection, stir, fully dissolve and mix, add 50ml of glycerin diluted with water to obtain a stock solution, add recombinant human growth Hormone stock solution and cholesterol, constant volume, to obtain liquid medicine; b. Under A-level environment, filter the liquid medicine obtained in step a through a 0.22 μm microporous filter membrane, and then pack to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com