Pd-Mg/C catalyst for preparing 2,3-dichloropyridine by catalytic hydrogenation of 2,3,6-trichloropyridine and preparation method of Pd-Mg/C catalyst
A technology of catalytic hydrogenation and trichloropyridine, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., and can solve the problems of limited industrial application, environmental pollution, and yield Low-level problems, to achieve the effect of improving reusable performance, reducing production costs, and enhancing interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
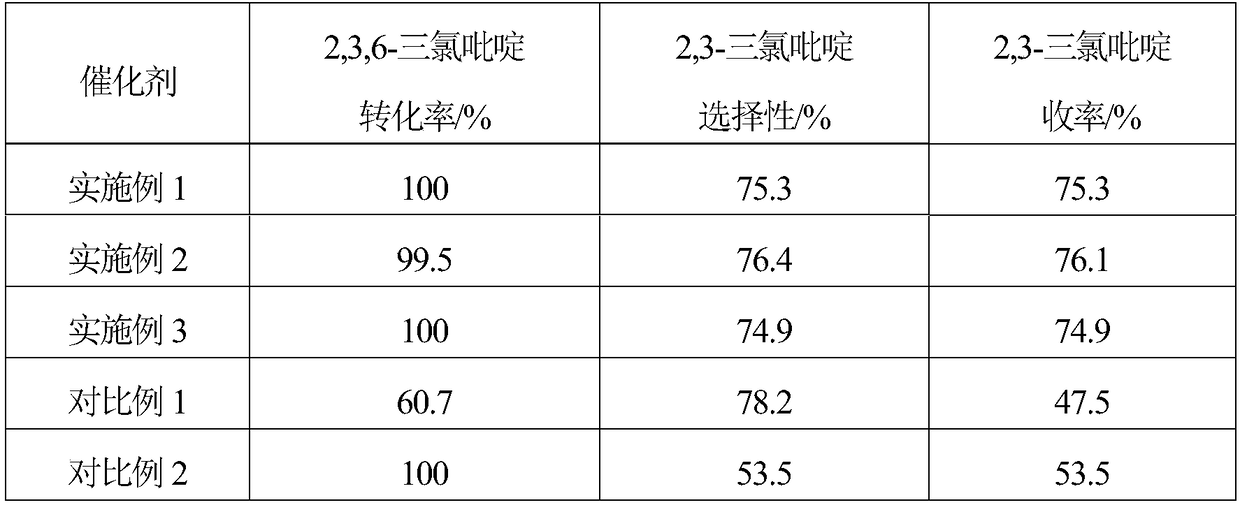
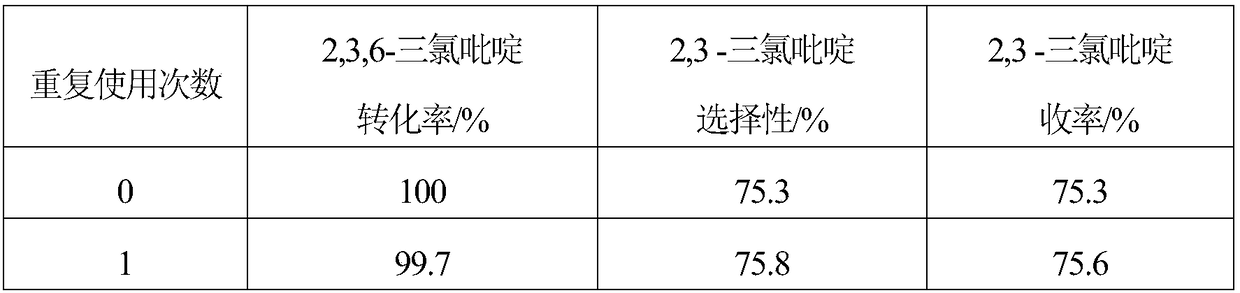
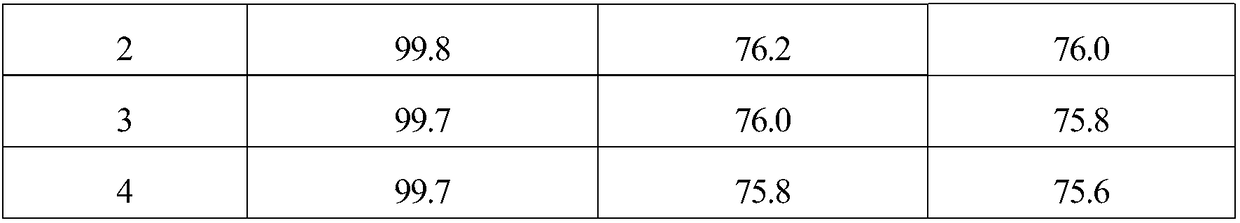
Embodiment 1
[0018] 1. The specific surface area of 10g is 1562m 2 / g of activated carbon is added to the mixed aqueous solution of 200mL hydrogen peroxide and hydrochloric acid, and the H in the mixed solution 2 o 2 The mass concentration is 10%, the HCl mass concentration is 5%, soaking at room temperature for 1 hour, then boiling for 20 minutes, washing with distilled water until neutral, and obtaining modified activated carbon.
[0019] 2. Mix 6.49g (2.82×10 -2 mol)Na 2 C 4 h 4 o 6 2H 2 Dissolve O in 200mL of distilled water, then add 9.5g of modified activated carbon, stir evenly, raise the temperature to 60°C, stir at constant temperature for 40 minutes, then add 20mL containing 1.38g Na 2 PdCl 4 (4.69×10 -3 mol) and 0.29g MgCl 2 ·6H 2 The aqueous solution of O was continuously stirred at 60°C for 2.5 hours, after standing for layers, the supernatant was poured off, and dried at 120°C.
[0020] 3. The dried sample was reduced in a hydrogen atmosphere at 200°C for 2 hour...
Embodiment 2
[0022] 1. The specific surface area of 10g is 1218m 2 / g of activated carbon is added to the mixed aqueous solution of 200mL hydrogen peroxide and hydrochloric acid, and the H in the mixed solution 2 o 2 The mass concentration is 5%, the HCl mass concentration is 5%, soaking at room temperature for 1 hour, then boiling for 20 minutes, washing with distilled water until neutral, and obtaining modified activated carbon.
[0023] 2. Mix 4.32g (1.88×10 -2 mol)Na 2 C 4 h 4 o 6 2H 2 Dissolve O in 200mL of distilled water, then add 9.5g of modified activated carbon, stir evenly, raise the temperature to 80°C, stir at constant temperature for 60 minutes, then add 20mL containing 1.38g Na 2 PdCl 4 (4.69×10 -3 mol) and 0.17g MgCl 2 ·6H 2 The aqueous solution of O was continuously stirred at 80°C for 3 hours, the supernatant liquid was decanted after standing for stratification, and dried at 120°C.
[0024] 3. The dried sample was reduced in a hydrogen atmosphere at 300°C f...
Embodiment 3
[0026] 1. The specific surface area of 10g is 1989m 2 / g of activated carbon is added to the mixed aqueous solution of 200mL hydrogen peroxide and hydrochloric acid, and the H in the mixed solution 2 o 2 The mass concentration is 15%, the HCl mass concentration is 5%, soaking at room temperature for 1 hour, then boiling for 20 minutes, washing with distilled water until neutral, and obtaining modified activated carbon.
[0027] 2. Mix 8.64g (3.75×10 -2 mol)Na 2 C 4 h 4 o 6 2H 2 Dissolve O in 200mL of distilled water, then add 9.5g of modified activated carbon, stir evenly, raise the temperature to 50°C, stir at constant temperature for 30 minutes, then add 20mL containing 1.38g Na 2 PdCl 4 (4.69×10 -3 mol) and 0.42g MgCl 2 ·6H 2 The aqueous solution of O was continuously stirred at 50°C for 2 hours, the supernatant liquid was decanted after standing for stratification, and dried at 120°C.
[0028] 3. The dried sample was reduced in a hydrogen atmosphere at 250°C ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com