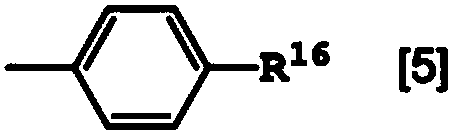
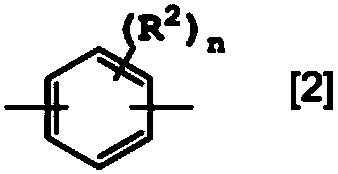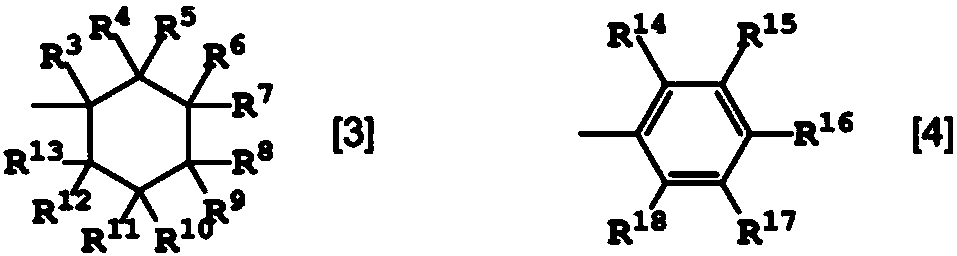Polyamide resin composition including carboxylic acid derivative
A polyamide resin and carboxylic acid derivative technology, applied in the field of polyamide resin composition, can solve the problems of damage to the mechanical properties of the appearance of molded products, softening, slow crystallization speed, etc., and achieve the effect of excellent transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] Hereinafter, although an Example is given and this invention is demonstrated more concretely, this invention is not limited to the following Example.
[0105] In addition, in the examples, the apparatus and conditions used for the preparation of the sample and the analysis of the physical properties are as follows.
[0106] (1) 5% weight loss temperature (Td 5% )
[0107] Device: Thermo plus EVO II TG8120 manufactured by Rigaku Co., Ltd.
[0108] Measuring conditions: under air atmosphere
[0109] Heating rate: 10°C / min (30~500°C)
[0110] (2) Differential scanning calorimetry (DSC)
[0111] Device: Diamond DSC manufactured by Perkin Elma Japan Co., Ltd.
[0112] (3) Total light transmittance
[0113] Device: Nippon Denshoku Industry Co., Ltd. haze meter NDH 5000
[0114] (4) Melt mixing
[0115] Device: ラボプラストミルマイクロ KF6V manufactured by Toyo Seiki Seisakusho Co., Ltd.
[0116] (5) hot pressing
[0117] Device: Tester Sangyo Co., Ltd. SA-302 bench type test pr...
manufacture example 1
[0124] [Manufacturing example 1] N 1,N 4 -Manufacture of bis(4-acetamidophenyl)succinamide
[0125] Into a reaction flask equipped with a stirrer, a thermometer, a dropping funnel, and a condenser, 3.00 g (20 mmol) of 4-aminoacetanilide [manufactured by Tokyo Chemical Industry Co., Ltd.], 2.02 g (20 mmol) of TEA, and 45.2 g of DMAc (relatively 9 times the total mass of 4-aminoacetanilide and TEA), cooled in an ice bath while stirring. To this solution, a solution obtained by dissolving 1.55 g (10 mmol) of succinoyl chloride [manufactured by Tokyo Chemical Industry Co., Ltd.] in 14.0 g of DMAc (9 times the mass of succinoyl chloride) was slowly added dropwise, Stirring was carried out for 3 hours. 3.6 g (200 mmol) of water was added dropwise to the reaction mixture to quench unreacted succinoyl chloride. The product was filtered under reduced pressure, washed with 100 g of a water-methanol mixed solution (mass ratio 3:7), and dried to obtain the target product (Compound A) ...
manufacture example 2
[0127] [Manufacturing example 2] N 1 ,N 5 -Manufacture of bis(4-acetamidophenyl)glutaramide
[0128] Separately, 1.69 g (10 mmol) of glutaryl chloride [manufactured by Tokyo Chemical Industry Co., Ltd.] was changed to succinoyl chloride, and the amount of water added in the post-treatment was changed to 30 g. In the same manner, the target product (compound B) was obtained as a white powder.
[0129] 5% weight reduction temperature (Td 5% ) is 342.4°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com