Preparation method of glucoside of flavonoid compound
A technology of flavonoids and glucosides, applied in the field of drug synthesis, can solve the problems of inconvenient use, unfriendly environment, low efficiency and the like, and achieve the effects of simple operation, environmental friendliness and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] In a preferred embodiment, the above preparation method further includes the step of separating and purifying the glucoside of the flavonoid aglycone. Examples of suitable chromatography include, but are not limited to, reverse phase column chromatography (RPC), high performance liquid chromatography (HPLC), gel permeation chromatography (GPC), supercritical fluid chromatography (SFC), high speed countercurrent chromatography method (HSCCC), ion exchange chromatography (IEC), thin layer chromatography (TLC), column chromatography (CC), etc. These means of separation and purification are conducive to further improving the purity of glucoside products.
[0024] In the context of the present invention, the term "aglycone" refers to a non-sugar substance capable of forming a glycoside compound through a dehydration reaction of its active hydrogen atom with a hemiacetal or hemiketal hydroxyl group of a sugar or a sugar derivative. Therefore, from the perspective of natural ...
Embodiment 1
[0053] Example 1: Simultaneous conversion of calycosin and formonetin.
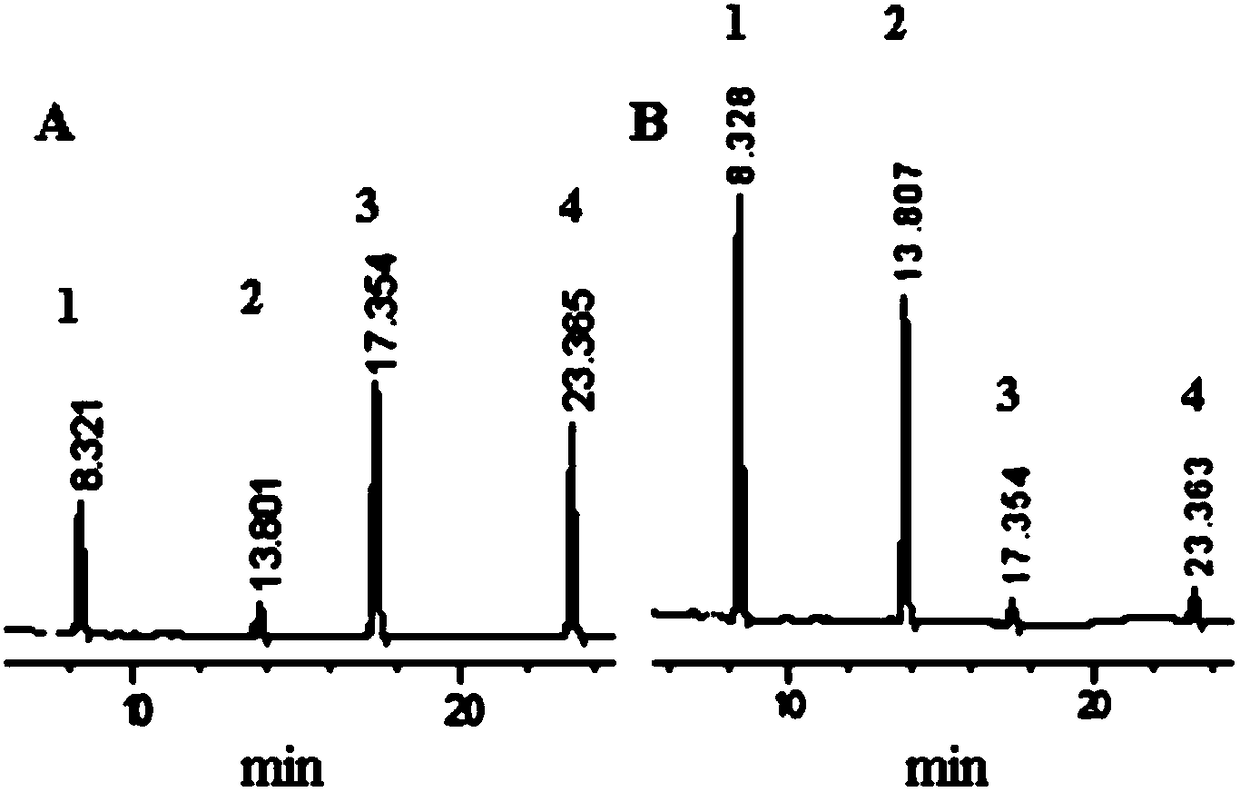
[0054] Measure 2mL of 20% (w / w) Vitex twig honey water, put it in a reaction container, and then add 10μL, 10μL, 5μL and 5μL of aqueous solutions of actecoisoflavone, formononetin, actecoside and formononetin, respectively , the concentration of honey in the resulting solution was about 200 mg / mL, while the concentrations of acteosin, formononetin, acteotin and formononetin were 7.38 μg / mL, 6.56 μg / mL, and 8.70 μg / mL, respectively and 2.07μg / mL, wherein the weight ratio of actecoisoflavone and honey is about 1:27000, the weight ratio of formononetin and honey is about 1:30000, and the molar ratio of actcoisoflavone and actcoisoflavone glycoside is about 1.4:1 , the molar ratio of formononetin and formononetin is about 0.5:1, vortex mix, seal, heat in a metal bath for 2h, the reaction temperature is 110 ℃, after the reaction is completed, cool to room temperature, through HPLC Analyzing conversion results...
Embodiment 2
[0060] Example 2: Simultaneous conversion of calycosin and formonetin.
[0061] Measure 4mL of 50% (w / w) Vitex twig honey water, put it in a reaction vessel, and then add 10 μL, 10 μL, 5 μL and 5 μL of aqueous solutions of actecosin, formononetin, actecoside and formononetin, respectively , the concentration of honey in the resulting solution was about 600 mg / mL, while the concentrations of acteosin, formononetin, acteoisoflavone glycoside and formononetin were 4.08 μg / mL, 5.18 μg / mL, and 9.06 μg / mL, respectively and 5.06 μg / mL, wherein the weight ratio of calycosin and honey is about 1:147000, the weight ratio of formononetin and honey is about 1:116000, and the molar ratio of calycosin and verbascoside is about 0.7:1 , the molar ratio of formononetin and formononetin is about 1.6:1, vortex mix, seal, heat in a metal bath for 0.5h, the reaction temperature is 100 ° C, after the reaction is completed, cool to room temperature, pass The conversion results were analyzed by HPLC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com