Granular type adjuvant as well as preparation method and application thereof
A granular and adjuvant technology, applied in the biological field, can solve the problems of ATRA light instability, reduced bioavailability, poor water solubility, etc., and achieve the effects of protecting from hydrolysis, promoting interaction, and delaying the release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
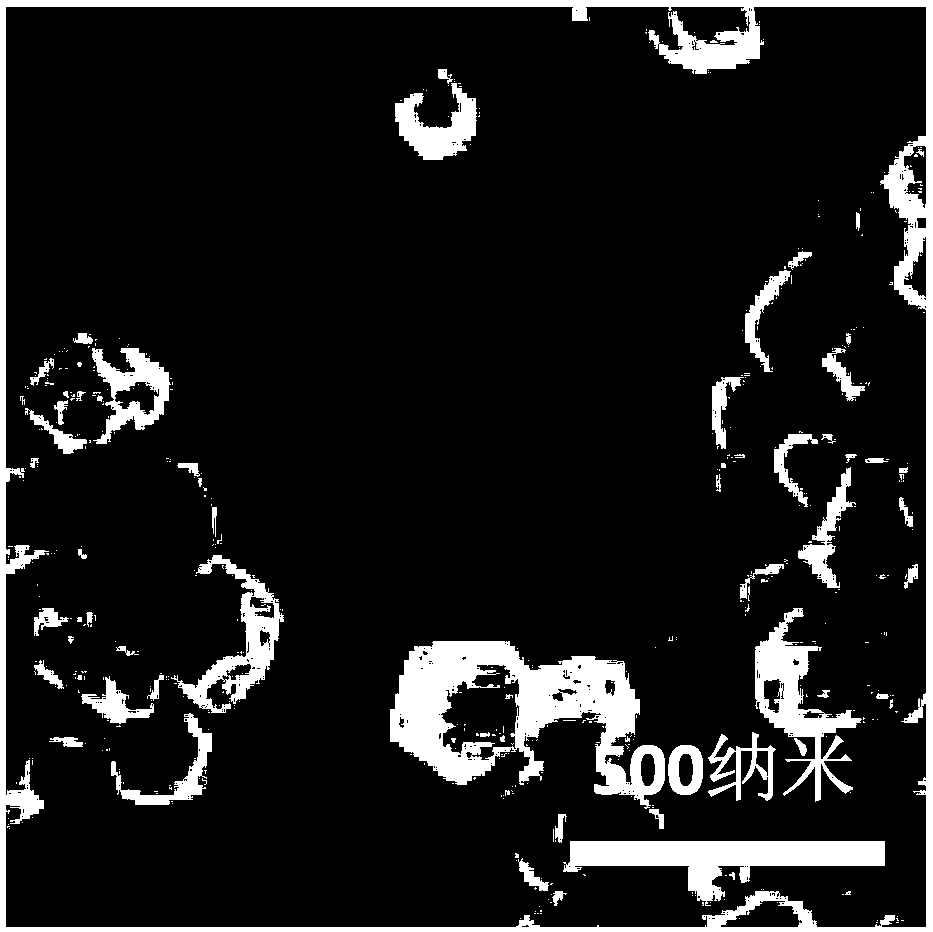
Image
Examples
Embodiment 1
[0131] In this example, ATRA-embedded biocompatible oil and biocompatible polymer material composite particle adjuvant was prepared by the following method
[0132] Accurately weigh 2.0 g of PLGA (molecular weight: 130,000 Daltons), 1.0 g of squalene, 0.3 g of dimethyl dioctadecyl ammonium bromide and 1 mg of all-trans retinoic acid with an electronic balance, and dissolve them in 30 mL In the mixed solution of ethanol, acetone and dichloromethane (1:1:9), the solution was quickly poured into 250mL aqueous solution (containing 1wt.% of PVA (alcoholysis degree is 99%, viscosity is 5.0mPa·s), Disperse under ultrasonic conditions (100W, 2min, interval time 4s). Stir overnight at 25°C (magnetic stirring, speed 500rpm) to remove the organic solvent in the system and achieve the curing effect. Centrifuge at 25000g for 10min, discard the supernatant , add 10 mL of deionized water to the precipitate, ultrasonically disperse, centrifuge at 25,000g for 10 min, discard the supernatant, f...
Embodiment 2
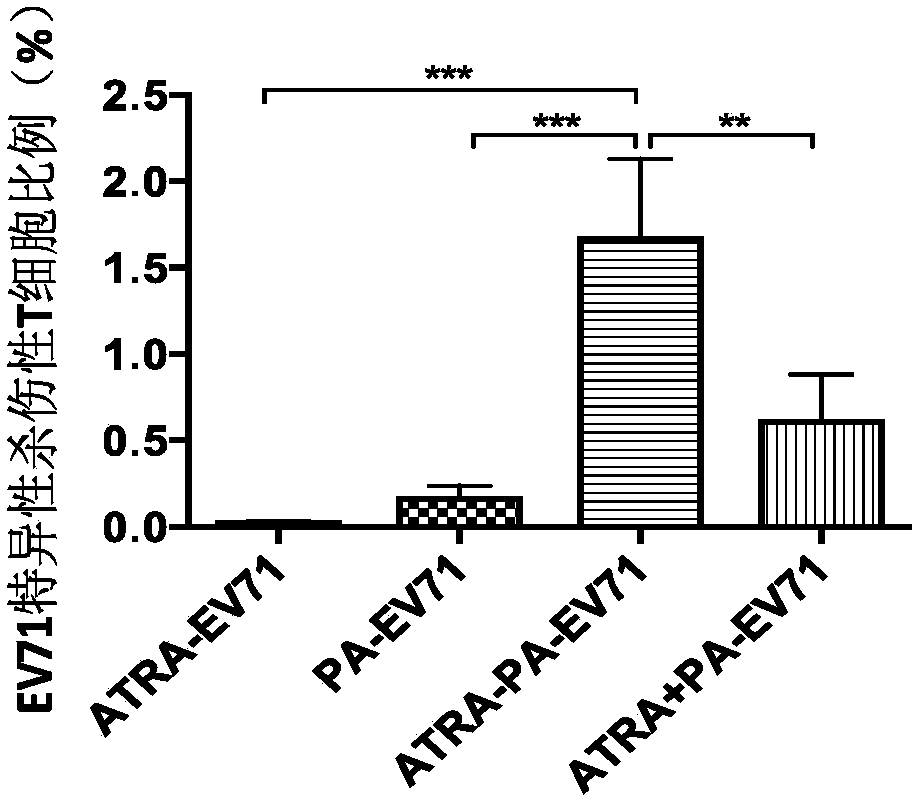
[0141] In this example, an ATRA-embedded particle adjuvant that triggers both intestinal mucosal and systemic immune responses as an EV71 recombinant vaccine adjuvant was prepared by the following method:
[0142]Adopt electronic balance to accurately weigh 1.0g of PLGA (molecular weight is 130,000 Daltons), 1.0g squalene, 0.2g dimethyl dioctadecyl ammonium bromide and 0.1mg all-trans retinoic acid, dissolve in 30mL of a mixed solution of ethanol and dichloromethane (1:9), quickly pour the solution into 200mL aqueous solution (containing 2wt.% of PVA (alcoholysis degree of 99%, viscosity of 8.0 mPa s), in the fast membrane Emulsification (the membrane pore size is 1.4 μm, the membrane pressure is 3MPa, and the membrane is passed 5 times). Stir overnight at 25°C (magnetic stirring, the rotation speed is 500rpm), and use the normal temperature solvent evaporation method to solidify the hard emulsion particles. Centrifuge at 15000g for 5min, discard Remove the supernatant, add 10...
Embodiment 3
[0151] In this example, ATRA-embedded particle adjuvants were prepared as HIV nucleic acid vaccine adjuvants to trigger a dual response of reproductive mucosa and systemic immunity:
[0152] Accurately weigh 2.0g of PLGA (molecular weight: 130,000 Daltons), 1.0g of ethyl linoleate / miglitol (1:1), and 0.2g of brominated dimethyl dioctadecyl using an electronic balance Ammonium and 0.1 mg of all-trans retinoic acid were dissolved in 20 mL of ethanol, a mixed solution of acetone and dichloromethane (1:2:7), and the solution was quickly poured into 100 mL of aqueous solution (containing 2wt.% of PVA (alcoholysis) degree of 99%, viscosity is 5.0mPa s), high-pressure homogeneous (10000rpm, 5.0MPa, 5min). Stir overnight at 25°C (magnetic stirring, rotating speed is 500rpm), thereby solidifying the particles. Centrifuge at 15000g for 10min, Discard the supernatant, add 10 mL of deionized water to the precipitate, ultrasonically disperse, and centrifuge at 15,000 g for 10 min. After di...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com