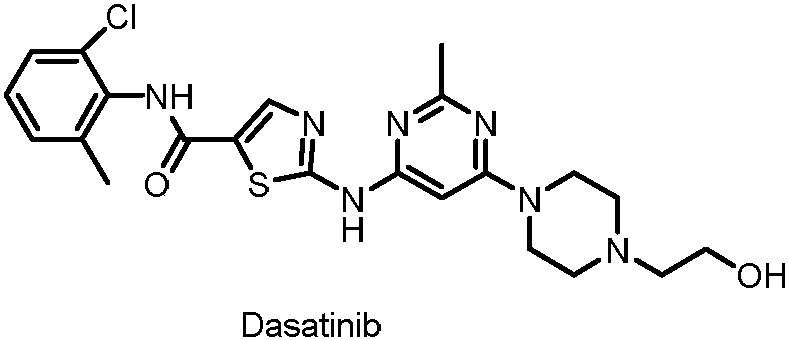
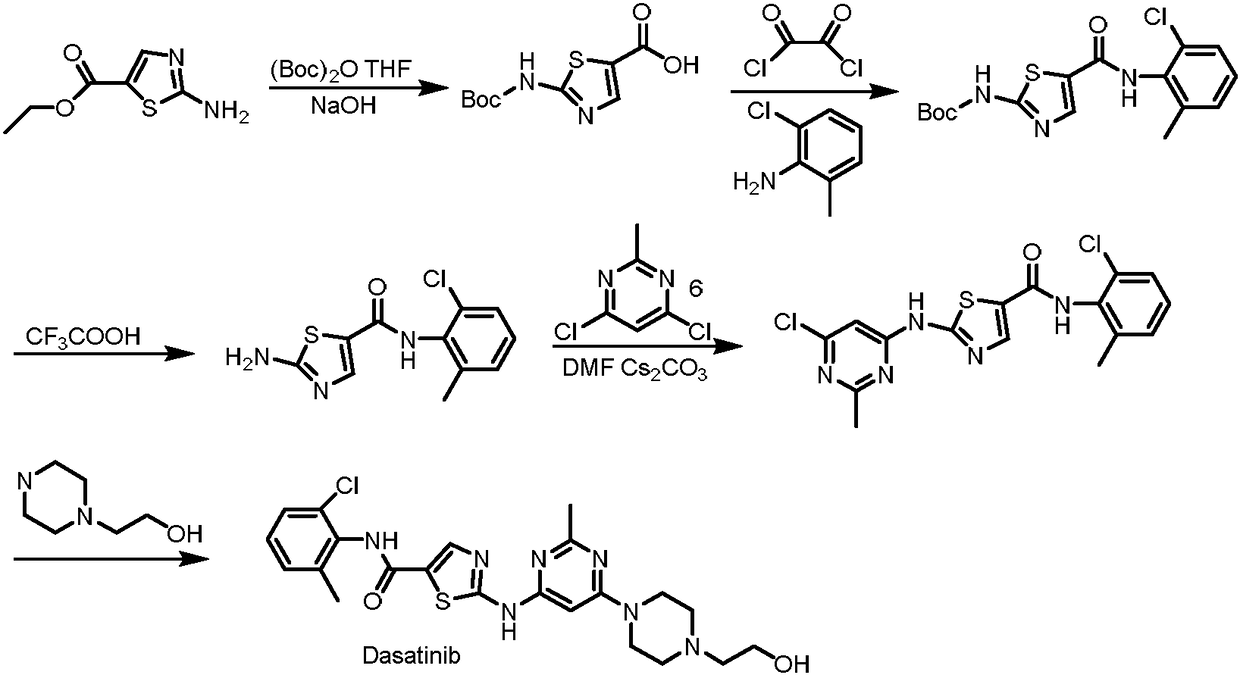
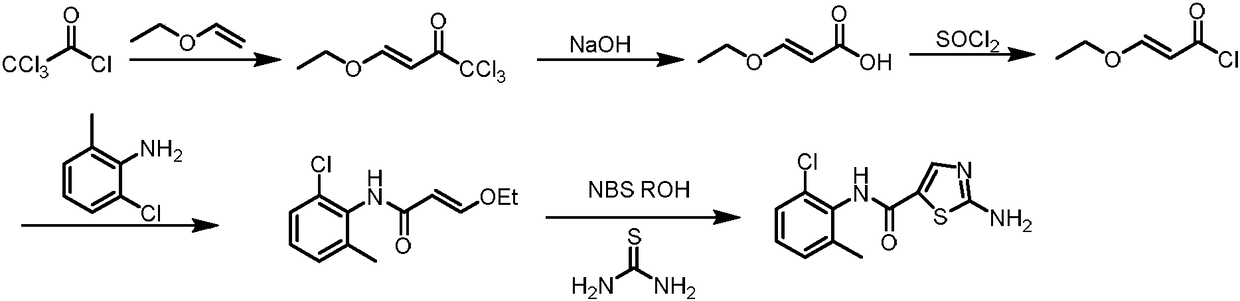
Synthesis method of dasatinib key intermediate
A synthesis method and technology of dasatinib, which are applied in the field of synthesis of key intermediates of dasatinib, can solve the problems affecting reaction selectivity, final yield, harsh conditions, and increased cost, and achieve the improvement of atom utilization, The effect of improving the reaction yield and reducing the workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (Compound 2)
[0041] At room temperature, 14.1g (100mmol) of 2-chloro-6-methylaniline and 14.7g (110mmol) of NCS were added to the flask, stirred and mixed in 80ml of acetonitrile, and reacted for 1.5h; then the temperature was raised to 60°C and the 5-methylthiazole 14.9g (150mmol), catalyst ferric chloride 1.6g (10mmol), oxidizer 60wt% tert-butyl hydroperoxide aqueous solution 27g (300mmol) and additive tetrabutylammonium iodide 3.7g (10mmol) were added to the mixture obtained by mixing The medium contact reaction for 5h, cool to room temperature, extract with dichloromethane, concentrate the organic phase, wash with water, then recrystallize from ethanol and dry to obtain N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (compound 2 ) 22.9g, the yield is 91%, and the HPLC purity is 99.72%.
Embodiment 2
[0043] Preparation of N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (Compound 2)
[0044] At room temperature, 14.1g (100mmol) of 2-chloro-6-methylaniline and 14.7g (110mmol) of NCS were added to the flask, stirred and mixed in 80ml of acetonitrile, and reacted for 1.5h; then the temperature was raised to 80°C and the 5-methylthiazole 14.9g (150mmol), catalyst ferric chloride 1.6g (10mmol), oxidizer 60wt% tert-butyl hydroperoxide aqueous solution 27g (300mmol) and additive tetrabutylammonium iodide 3.7g (10mmol) were added to the mixture obtained by mixing The medium contact reaction for 5h, cool to room temperature, extract with dichloromethane, concentrate the organic phase, wash with water, then recrystallize from ethanol and dry to obtain N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (Compound 2 ) 23.9g, the yield is 95%, and the HPLC purity is 99.65%.
Embodiment 3
[0046] Preparation of N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (Compound 2)
[0047] At room temperature, 14.1g (100mmol) of 2-chloro-6-methylaniline and 14.7g (110mmol) of NCS were added to the flask, stirred and mixed in 80ml of acetonitrile, and reacted for 1.5h; then the temperature was raised to 90°C and the 5-methylthiazole 14.9g (150mmol), catalyst ferric chloride 1.6g (10mmol), oxidizer 60wt% tert-butyl hydroperoxide aqueous solution 27g (300mmol) and additive tetrabutylammonium iodide 3.7g (10mmol) were added to the mixture obtained by mixing The medium contact reaction for 5h, cool to room temperature, extract with dichloromethane, concentrate the organic phase, wash with water, then recrystallize from ethanol and dry to obtain N-(2-chloro-6-methylphenyl)thiazole-5-carboxamide (Compound 2 ) 23.68g, the yield is 94%, and the HPLC purity is 99.76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com