Prodrugs of glutamine analogs
A technology of glutamine and analogs, applied in the field of prodrugs of glutamine analogs, can solve problems such as hindering clinical development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0310] method
[0311] General procedure: Commercially available HPLC grade methanol, catalyst and reagent grade materials were used as received. TLC was carried out on a silica gel 60F254 coated aluminum plate (Merck), and the 4 ) 2 Solution detection spot. 4H 2 O (1%) and H 3 P(Mo 3 o 10 ) 4 (2%) in sulfuric acid (10%). Use Isolera One HPFC system (Biotage company) on silica gel 60 (0.040-0.063mm, Fluka) or on KP‐C18‐HS or KP‐ Flash chromatography was performed on a SNAP cartridge. All chemicals were purchased from Sigma-Aldrich and used without further purification. Measured at 400.1MHz, 500.1MHz or 600.1MHz 1 H NMR spectrum, measured at 100.8MHz, 125.7MHz or 150.9MHz 13 C NMR spectrum. for 1 Normalization of H NMR spectra using the internal signal of TMS (δ0.0, CDCl 3 ) or the residual signal of the solvent (for CDCl 3 is δ7.26, for CD 3 COCD 3 is δ2.05, for CD 3 OD is δ3.31). exist 13 In the case of the C spectrum, use the residual signal of the ...
Embodiment 2
[0328] Prodrug Strategies - Masking of Carboxylate Functional Groups
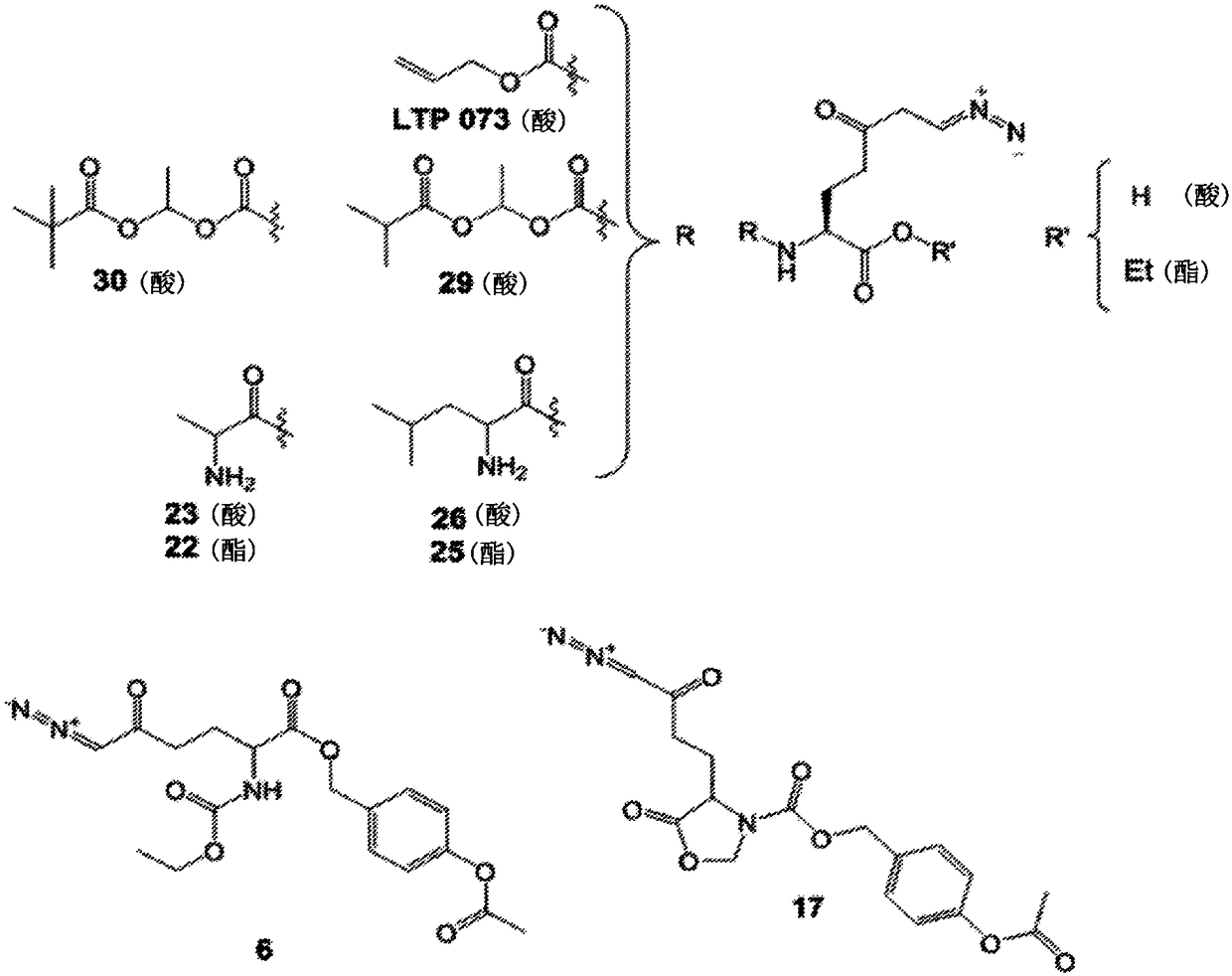
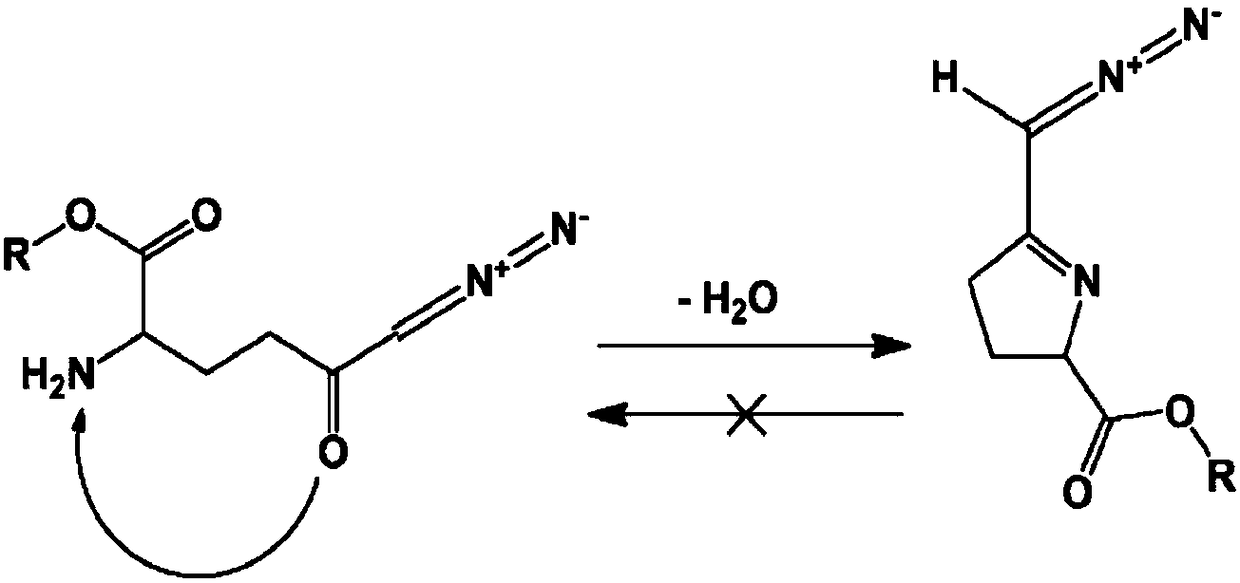
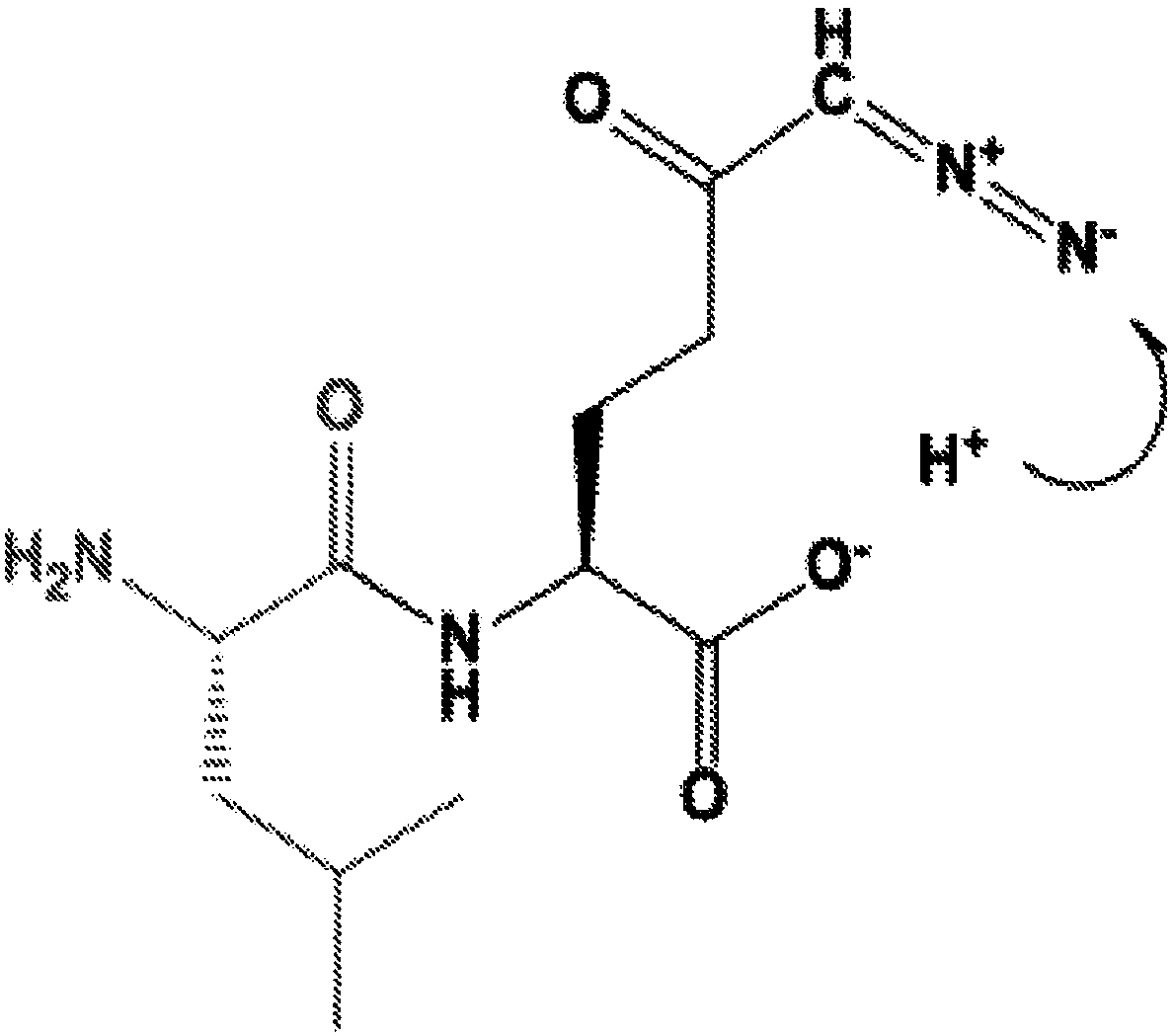
[0329] In one embodiment, DON prodrugs are designed by masking only the carboxylate functionality with an alkyl ester of DON with an unprotected α-amino group. However, some DON alkyl esters with unprotected α-amino functional groups were found to undergo cyclization to form 5-membered cyclic Schiff's bases. The observed cyclization is pH dependent and rapid at pH 5-7. At lower pH, which generally prevents or reverses cyclization, the diazo function becomes unstable. As a result, cyclization is virtually irreversible, making some N-α-free alkyl esters unsuitable as DON prodrugs ( figure 1 A).
Embodiment 3
[0331] Prodrug Strategies - Masking Amino Functional Groups
[0332] In another embodiment, DON prodrugs are designed by using N-protected derivatives of DON with unprotected carboxyl functionality to mask only the amino functionality. N-protected derivatives of DON with unprotected carboxyl functional groups ( figure 1 B and figure 1 C) is also unstable. More specifically, the acidic carboxyl functionality causes progressively slower decomposition of the diazo group. In some salt forms, the carboxylate anion destabilizes the N-alpha-protecting group. Going a step further, as in figure 1 D. figure 1 E. figure 1 F. figure 1 G and figure 1 As shown in H, many of the tested prodrugs (except 26) with free carboxylate had negligible exposure when administered orally compared to DON, further suggesting that derivatized carboxylate and amine functional groups are important for oral availability benefits.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com