Methods for cancer and immunotherapy using glutamine analogs
A technology for immunotherapy and cancer, applied in the direction of medical raw materials, drug combinations, and pharmaceutical formulations derived from mammals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
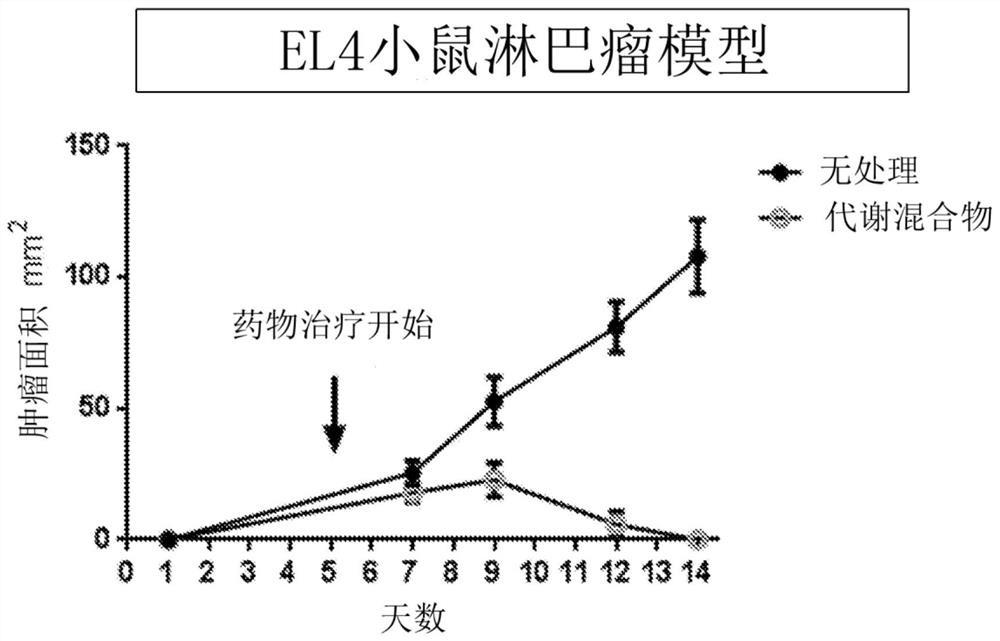
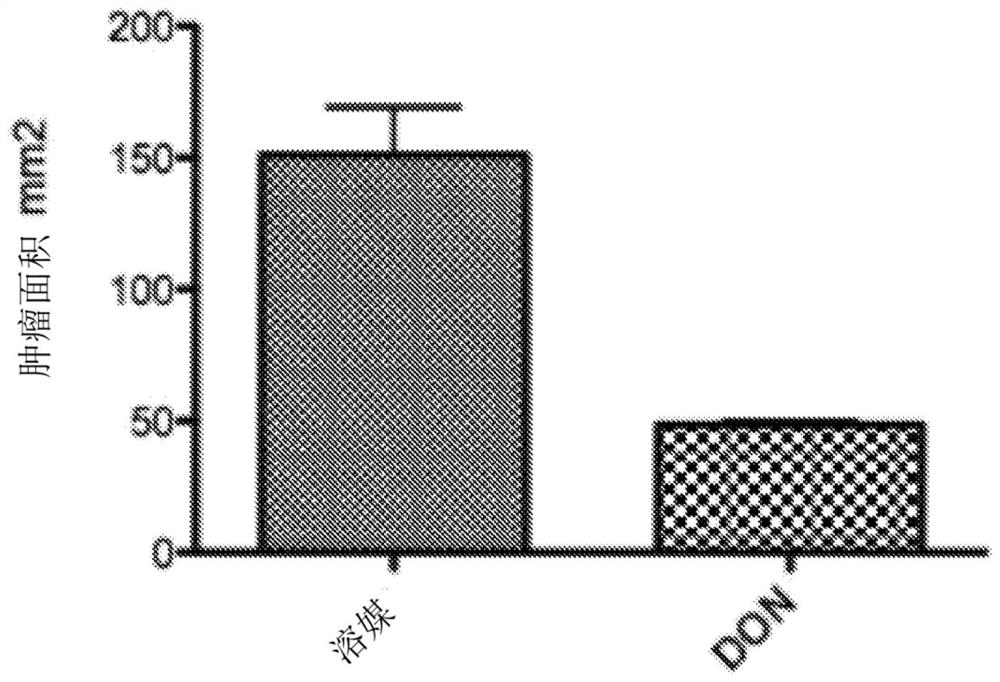
[0447] To study the effect of DON on cancer, the EL4 mouse lymphoma model was used, and it showed that DON can significantly inhibit the growth of lymphoma, indicating that bone marrow-derived tumors may be very sensitive to DON ( figure 1 ). However, DON had a modest effect on inhibiting the growth of melanomas that were not bone marrow-derived tumors ( figure 2 ).
Embodiment 2
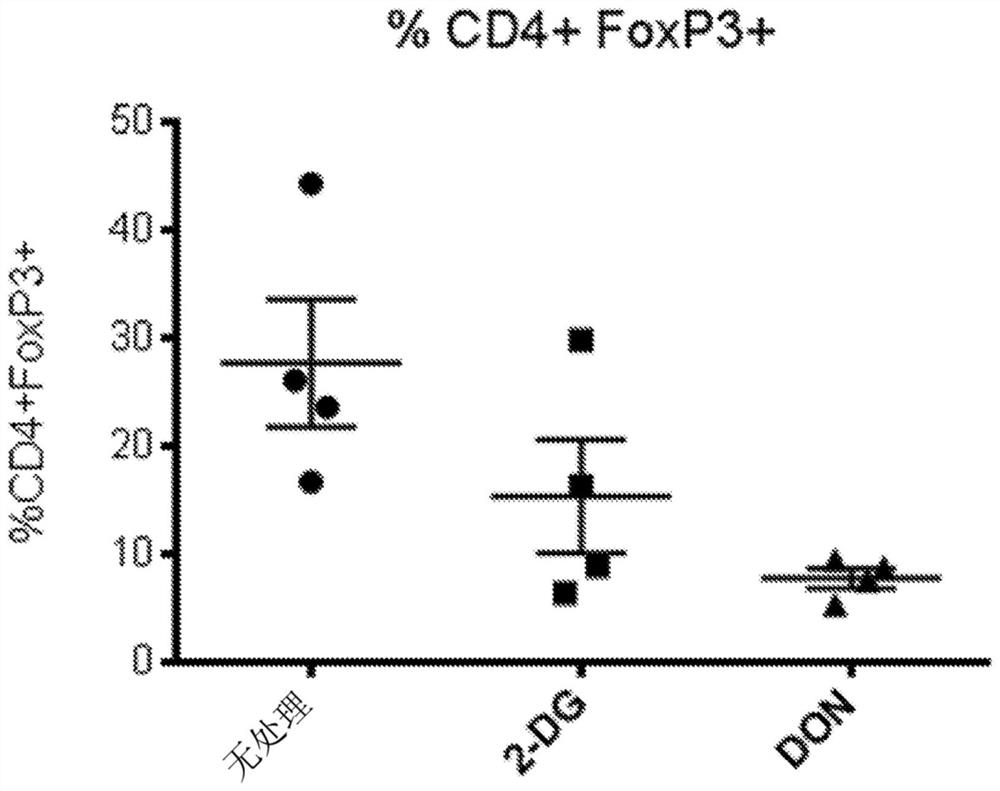
[0449] image 3 DON was shown to suppress tumor-infiltrating regulatory T cells (Foxp3 + ) to opsonize B16 melanoma to be killed by immunotherapy.
Embodiment 3
[0451] Summarize
[0452] The glutamine antagonist 6-diazo-5-oxo-L-norleucine (DON, 1) showed strong anticancer efficacy in preclinical and clinical studies but was discontinued due to significant systemic toxicity its development. Here, we demonstrate that DON inhibits glutamine metabolism and provides antitumor efficacy in a mouse glioblastoma model, despite observed toxicity. To increase the therapeutic index of DON, we used a prodrug strategy to increase its brain delivery and limit systemic exposure. Although these bipartite prodrugs exhibit rapid metabolism in mouse plasma, several bipartite prodrugs provided excellent stability in monkey and human plasma. The most stable compound (5c, methyl-POM_DON-isopropyl ester) was evaluated in monkeys where the brain:plasma ratio was increased 10-fold compared to DON. This strategy may provide an avenue for the utilization of DON in GBM patients.
[0453] introduce
[0454] Glioblastoma multiforme (GBM) is the most common and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com