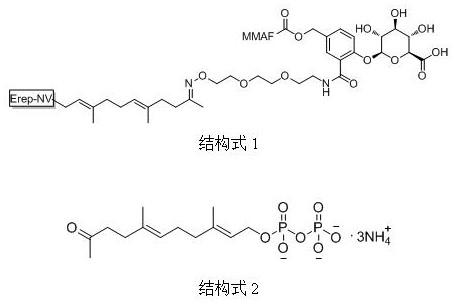
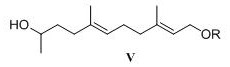
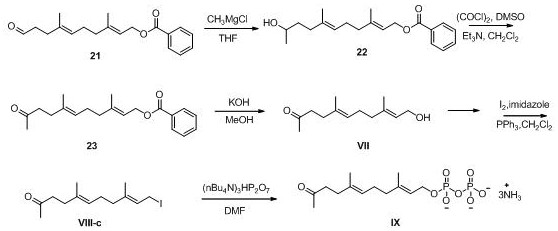
A kind of preparation method of acetonyl geranyl pyrophosphate intermediate
An oxidation system, iodoyl benzoic acid technology, applied in the field of medicine and chemical industry, can solve problems such as configuration impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0072] Benzoyl-protected hydroxyaldehydes 21
[0073]
[0074] Step 1 Preparation of acacia benzoate 11
[0075] Farnesol (15.0 g, 67 mmol) was dissolved in 120 ml of pyridine, cooled at low temperature to keep the inner temperature below 0 °C, benzoyl chloride (11.4 g, 81 mmol) was added dropwise, and the mixture was stirred at room temperature for 3 hours. The reaction solution was diluted with water, then extracted with petroleum ether three times, the petroleum ether layer was dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated and purified by column chromatography to obtain 20.1 g of the product with a yield of 90%.
[0076] Step 2 Preparation of epoxide 12
[0077] The farnesyl benzoate (20.0 g, 61 mmol) obtained in the previous step was dissolved in 240 ml of tetrahydrofuran, 120 ml of water was added, the internal temperature was kept below 0 °C by low temperature cooling, and N-bromosuccinimide ( NBS) (12.0 g, 67.4 mm...
preparation example 2
[0084] p-Methoxybenzyl (PMB) protected hydroxyaldehyde 24
[0085]
[0086] Step 1 Preparation of Acacia p-methoxybenzyl ether 9
[0087] Dissolve farnesol (5.0 g, 22.5 mmol) in 50 ml of N,N-dimethylformamide, cool at low temperature to make the inner temperature below 0 °C, add 60% sodium hydrogen (1.8 mg, 45 mmol), stir for 0.5 hour After that, p-methoxybenzyl chloride (PMBCl) (5.3 g, 34 mmol) was added dropwise, and the mixture was stirred at room temperature overnight. The reaction solution was diluted with water, then extracted with petroleum ether three times, the petroleum ether layer was dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and separated and purified by column chromatography to obtain 7.1 g of the product with a yield of 92%.
[0088] Step 2 Preparation of Epoxide 10
[0089] The farnesol p-methoxybenzyl ether (7.0 g, 20.4 mmol) obtained in the previous step was dissolved in 80 ml of tetrahydrofuran, 40 ml of water wa...
preparation example 3
[0094] tert-Butyldiphenylsilyl (TBDPS) protected hydroxyaldehyde 18
[0095]
[0096] Step 1 Preparation of Acacia pure TBDPS ether 7
[0097] Dissolve farnesol (10.0 g, 45.0 mmol) in 100 ml of dichloromethane, cool at low temperature to keep the internal temperature below 0 °C, then add tert-butyldiphenylchlorosilane (TBDPS) (16.0 g, 58.5 mmol), Then imidazole (5.0 g, 67.5 mmol) was added in portions, the addition was complete, the cooling bath was removed, and the mixture was stirred at room temperature for 1 hour. The reaction solution was washed successively with 100 ml of water and 100 ml of saturated aqueous sodium chloride solution. The dichloromethane layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to obtain 24.3 g of a light yellow transparent liquid, which was directly used in the next reaction.
[0098] Step 2 Preparation of Epoxide 8
[0099] Dissolve the farnesol TBDPS ether (24.3 g, 45 mmol) obtained in the pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com