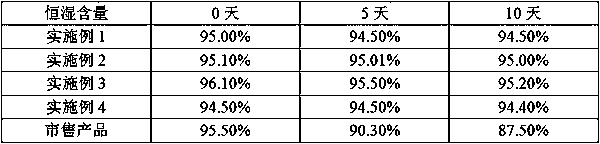
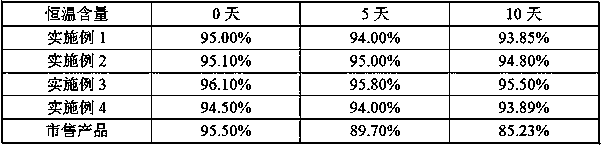
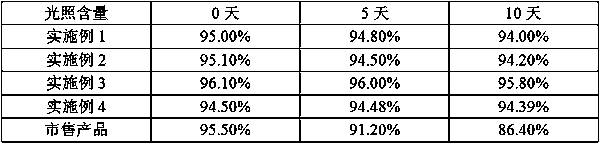
Method for preparing ranitidine hydrochloride capsules
A technology of ranitidine hydrochloride and capsules, which is applied in the field of preparation and production of ranitidine hydrochloride capsules, can solve the problems of short storage time, toxic and side effects of auxiliary materials, weak stability, etc. The intake of excipients, the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Weigh 5.0g ranitidine hydrochloride and 5.0g tapioca starch, grind them in a mortar respectively, and pass through an 80-mesh sieve to obtain a uniform powder, mix tapioca starch and ranitidine hydrochloride powder, grind for 30min, and after grinding for 10min, Put it in a microwave oven, heat it with 700W microwave for 5min, and microwave it twice to get a uniform medicinal powder, put the medicinal powder into No. 0 hydroxypropyl starch capsules, and fill each capsule with 50mg medicinal powder to prepare 100 capsules.
Embodiment 2
[0018] Weigh 1.0 g of ranitidine hydrochloride and 5.0 g of tapioca starch, grind them in a mortar respectively, and pass through an 80-mesh sieve to obtain a uniform powder, mix tapioca starch and ranitidine hydrochloride powder, and grind for 30 minutes, and after grinding for 10 minutes, Put it in a microwave oven, heat it with 700W microwave for 5min, and microwave it twice to get a uniform medicinal powder, put the medicinal powder into No. 0 hydroxypropyl starch capsules, and fill each capsule with 50mg medicinal powder to prepare 100 capsules.
Embodiment 3
[0020] Take by weighing 1.0g ranitidine hydrochloride, 10.0g tapioca starch and potato starch mixture, put into mortar respectively and grind, cross 80 mesh sieves, obtain uniform powder, mix tapioca starch and ranitidine hydrochloride powder, grind 30min, After grinding for 10 minutes, put it into a microwave oven, heat it with 1200W microwave for 5 minutes, and microwave it twice to obtain a uniform medicinal powder. Put the medicinal powder into No. 0 hydroxypropyl starch capsules, and fill each capsule with 50 mg of medicinal powder to prepare 100 capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com