Covalent organic framework material and preparation method thereof
A technology of covalent organic framework and reactive monomers, applied in separation methods, chemical instruments and methods, and other chemical processes, can solve the problems of volatile organic compound emissions, long reaction time, and complicated operation, and achieve convenient recycling and reuse , short reaction time, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
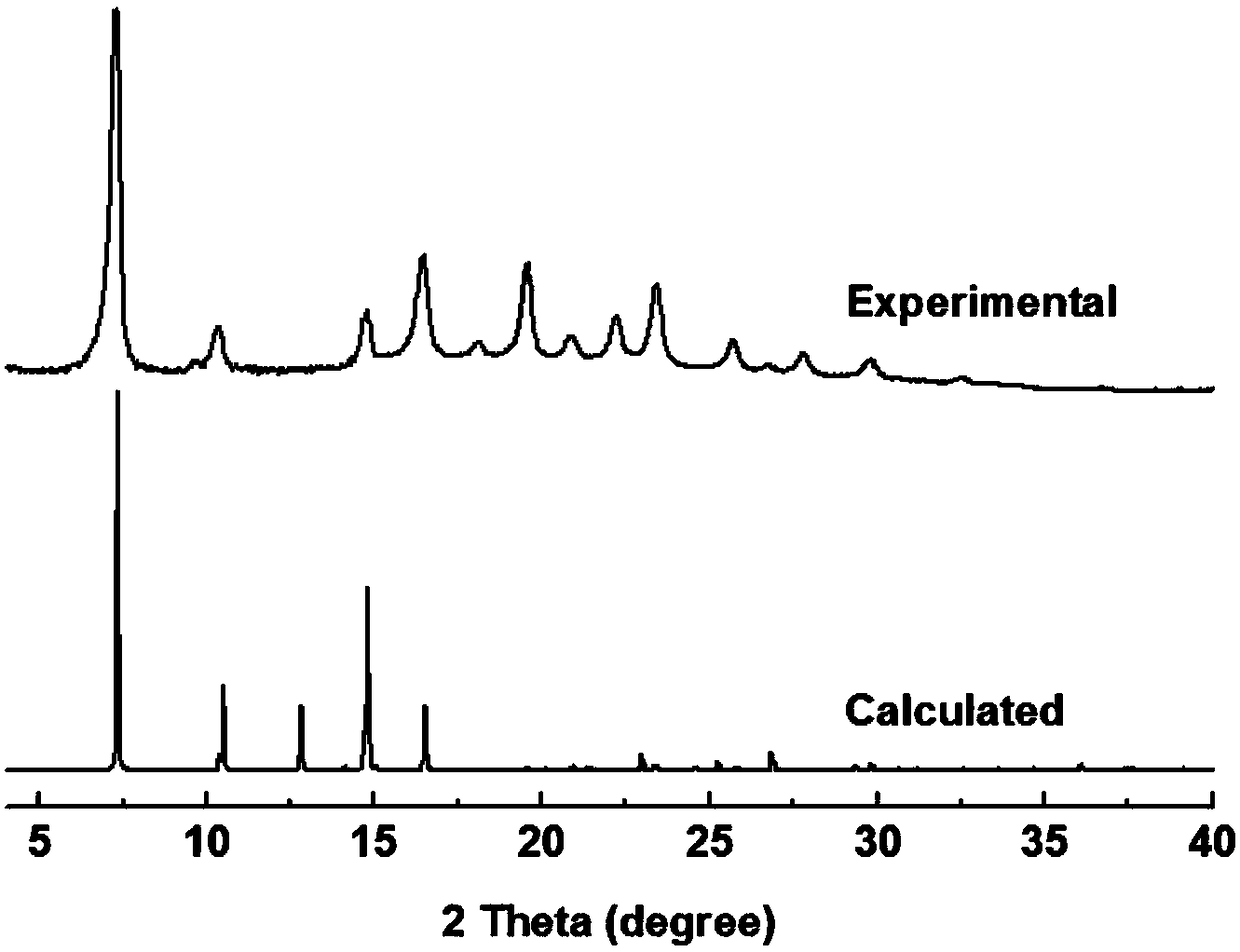
[0042] Example 1 Preparation of 3D-IL-COF-1 covalent organic framework material by 1-butyl-3-methylimidazole bistrifluoromethanesulfonimide salt
[0043] Weigh 21.6 mg of tetrakis(formylphenyl)methane and 10.8 mg of p-phenylenediamine, grind them evenly, add the mixture into a 5 mL centrifuge tube, add 100 μL of 1-butyl-3-methylimidazole bistrifluoromethanesulfonyl Mix the amine salts evenly, keep the reaction at room temperature for 3 minutes, wash and dry with ethanol and acetone after the reaction, and obtain a light yellow powder.
[0044] Its product XRD and simulated powder XRD diffraction patterns are as follows figure 1 , it can be seen that the simulated spectrum and the product spectrum are consistent.
Embodiment 2
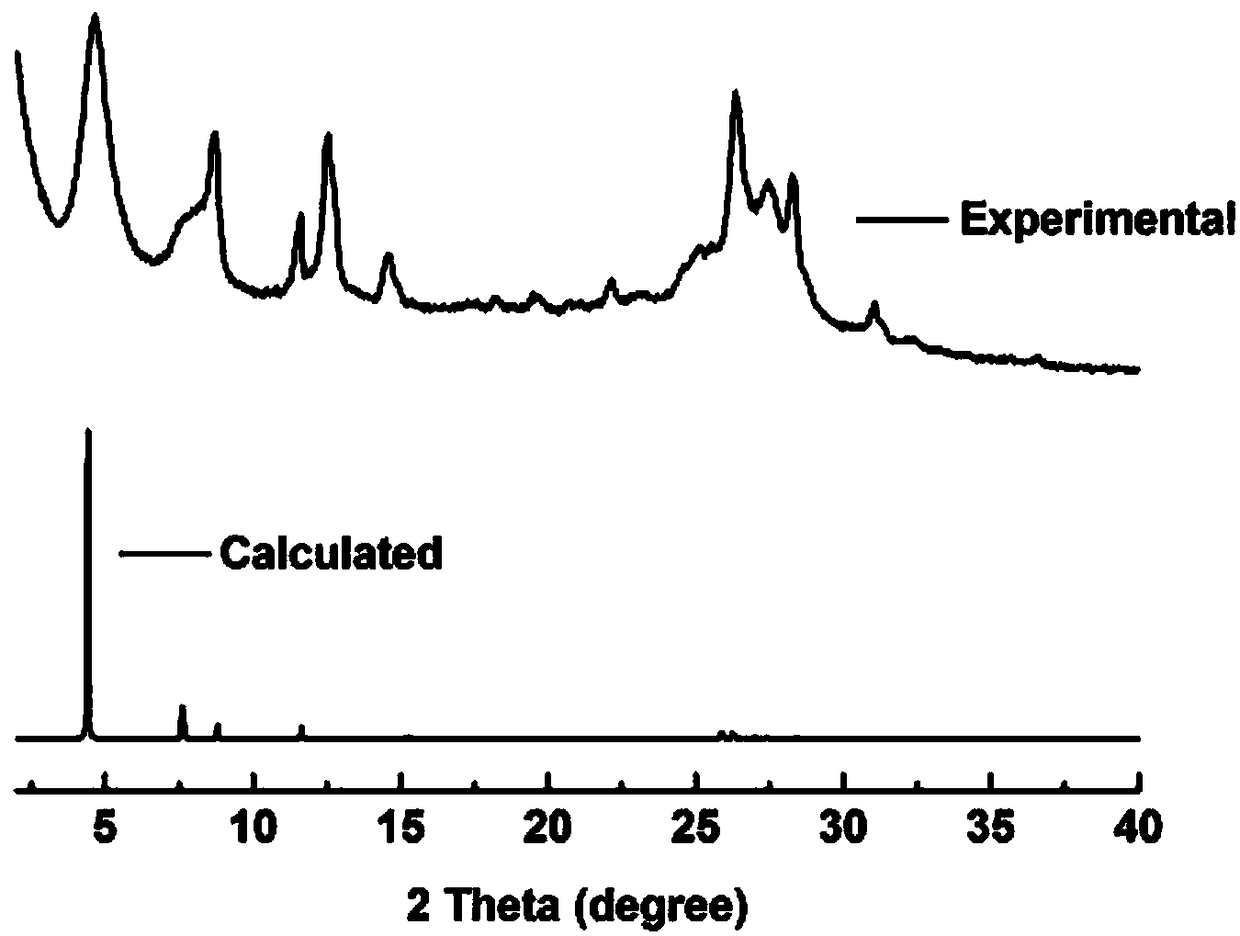
[0045] Example 2 Preparation of 2D-IL-COF-1 covalent organic framework material by 1-butyl-3-methylimidazole bistrifluoromethanesulfonimide salt
[0046] Weigh 14.0mg of 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde and 10.8mg of p-phenylenediamine, grind them evenly, add the mixture into a 5mL centrifuge tube, add 100μL of 1-butyl-3-formaldehyde The imidazole bis-trifluoromethanesulfonimide salt was mixed uniformly and kept at room temperature for 12 hours. After the reaction, it was washed and dried with acetone to obtain an orange-yellow powder.
[0047] Its product XRD and simulated powder XRD diffraction patterns are as follows figure 2 , it can be seen that the simulated spectrum and the product spectrum are consistent.
Embodiment 3
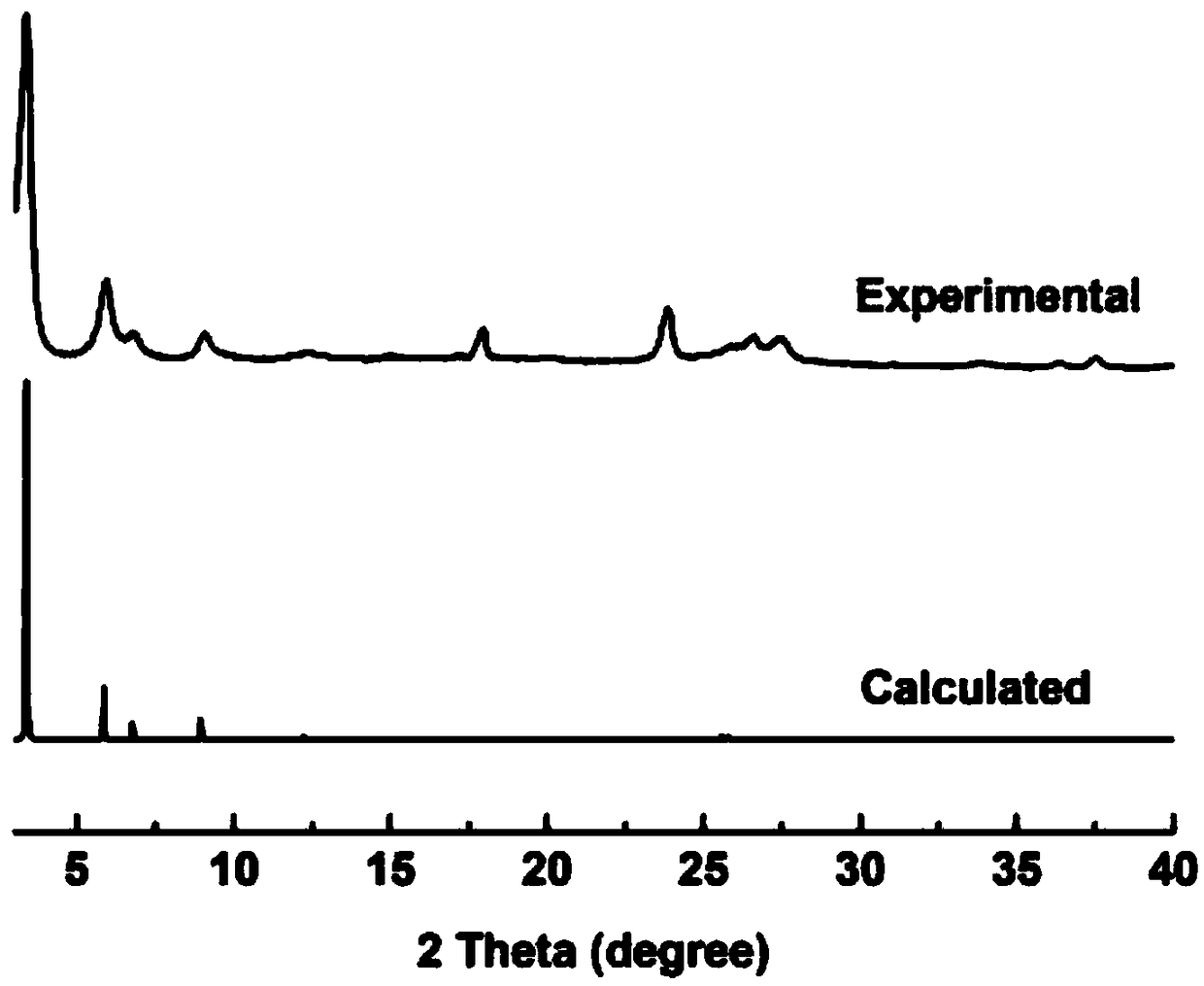
[0048] Example 3 Preparation of 2D-IL-COF-2 covalent organic framework material by 1-butyl-3-methylimidazole bistrifluoromethanesulfonimide salt
[0049] Weigh 25.0mg 1,4-terephthalic diboronic acid and 16.0mg 2,3,6,7,10,11-hexahydroxytriphenylene, grind evenly, add the mixture into a 5mL centrifuge tube, add 100μL 1-butyl- 3-Methylimidazole bistrifluoromethanesulfonyl imide salt, mixed evenly, kept at 60°C for 12 hours, washed with acetone and dried after the reaction to obtain a light gray powder.
[0050] Its product XRD and simulated powder XRD diffraction patterns are as follows image 3 , it can be seen that the simulated spectrum and the product spectrum are consistent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com