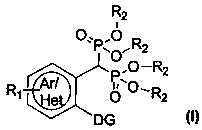
Preparation method and application of aryl bisphosphonate derivative
A technology of aryl geminal diphosphoric acid and derivatives, which is applied in the fields of medicinal chemistry and drug therapy, and achieves the effects of good inhibitory activity and clear efficacy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Synthesis of compound 1
[0034]
[0035] Add 0.3mmol of 2-phenylpyridine and 0.36mmol of tetraethyl methylene diphosphate to a dry sealed tube of 15ml, the catalyst dichloro(pentamethylcyclopentadienyl)rhodium(III) dimer (CAS No. 12354-85-7) 5% mol, silver sulfate 10% mol and solvent DCE 3ml, stirred at 80°C for 12 hours. After the reaction was completed, it was concentrated under reduced pressure, column chromatography, DCM / MeOH (80:1) to obtain a yellow oily product, and the yield was 93%.
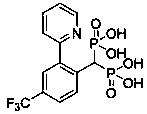
[0036] 1 H NMR (400 MHz, CDCl 3 ) δ 8.69 (d, J = 4.5 Hz, 1H), 8.03 (d, J = 7.5Hz, 1H), 7.77 (td, J = 7.7, 1.4 Hz, 1H), 7.54 (d, J = 7.8 Hz, 1H), 7.48 –7.34 (m, 3H), 7.32 – 7.20 (m, 1H), 5.09 (t, J = 25.7 Hz, 1H), 4.29 – 3.87 (m,8H), 1.21 (t, J = 7.0 Hz, 6H), 1.15 (t, J = 7.0 Hz, 6H). 13 C NMR (150 MHz, CDCl 3 ( J =130.65Hz),16.25, 16.21, 16.17, 16.13; 31 P NMR (162 MHz, CDCl 3 ) δ 19.45.
Embodiment 2
[0038] Synthesis of compound 2
[0039]
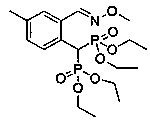
[0040] Add 0.3mmol of o-methyl 2-phenylpyridine and 0.36mmol of tetraethyl methylene diphosphate to a dry sealed tube of 15ml, and the catalyst dichloro(pentamethylcyclopentadienyl)rhodium(III) Dimer (CAS No. 12354-85-7) 5% mol, silver sulfate 10% mol and solvent DCE 3ml, stirred at 80°C for 12 hours. After completion of the reaction, concentrated under reduced pressure, column chromatography, DCM / MeOH (80:1) to obtain 98.4 mg of yellow oil product, yield 72%, 1 H NMR (400 MHz, CDCl 3) δ 8.71 (d, J =4.5 Hz, 1H), 7.82 (d, J = 7.7 Hz, 1H), 7.75 (dd, J = 10.8, 4.5 Hz, 1H), 7.39(d, J = 7.7 Hz, 1H), 7.34 – 7.28 (m, 2H), 7.21 (d, J = 7.5 Hz, 1H), 4.04(ddt, J = 23.8, 16.1, 8.0 Hz, 8H), 3.72 (t, J = 25.4 Hz, 1H), 2.02 (s, 3H), 1.24 (t, J = 5.8 Hz, 6H), 1.18 (t, J = 7.0 Hz, 6H); 13 C NMR (150 MHz, CDCl 3 ) Δ158.25, 149.60, 136.73, 136.12, 129.48, 127.83, 127.77, 127.74, 125.57,122.14, 112.47, 63.38, 62.79, 62.74, 41.45 ...
Embodiment 3
[0042] Synthesis of compound 3
[0043]
[0044] Add 0.3mmol of o-methoxy 2-phenylpyridine and 0.36mmol of tetraethyl methylene diphosphate to a dry 15ml sealed tube, and the catalyst dichloro(pentamethylcyclopentadienyl) rhodium (III ) dimer (CAS No. 12354-85-7) 5% mol, silver sulfate 10% mol and solvent DCE 3ml, stirred at 80°C for 12 hours. After completion of the reaction, concentrated under reduced pressure, column chromatography, DCM / MeOH (80:1) to obtain 96.2 mg of yellow oil product, yield 68%,. 1 H NMR (400 MHz, DMSO- d 6 ) δ 8.65(d, J = 4.4 Hz, 1H), 7.85 (t, J = 7.4 Hz, 1H), 7.45 – 7.31 (m, 4H), 7.08 (d, J = 7.5 Hz, 1H), 3.85 (ddd, J = 24.3, 15.2, 7.5 Hz, 8H), 3.67 (s, 3H), 1.12(t, J = 7.0 Hz, 6H), 1.02 (t, J = 7.0 Hz, 6H); 13 C NMR (100 MHz, CDCL 3 ( J = 136.80 Hz), 16.32; 31 P NMR (162MHz, DMSO- d 6 ) δ 18.69.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com