Mercury ion fluorescent probe based on rhodamine b and its preparation method and application
A fluorescent probe and mercury ion technology, applied in the field of mercury ion fluorescent probes, can solve the problems of complex preparation steps, poor water solubility and selectivity, and harsh test conditions of mercury ion fluorescent probes, and achieve relatively long absorption and emission wavelengths , high selectivity, and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
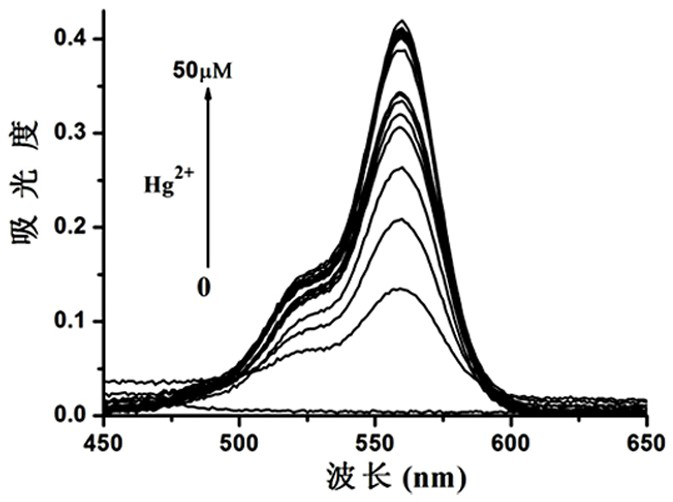
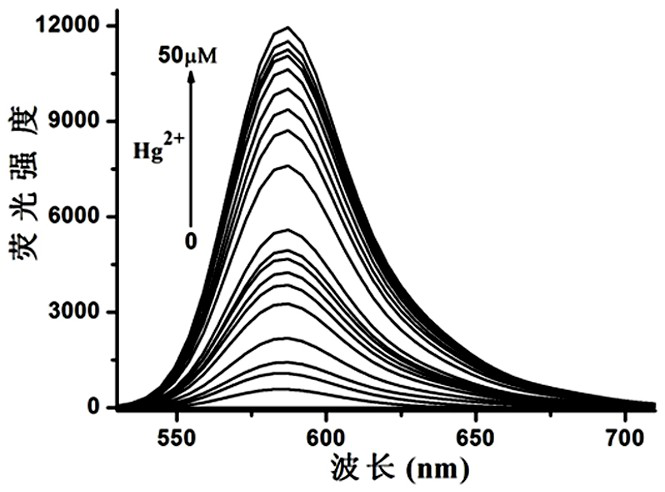
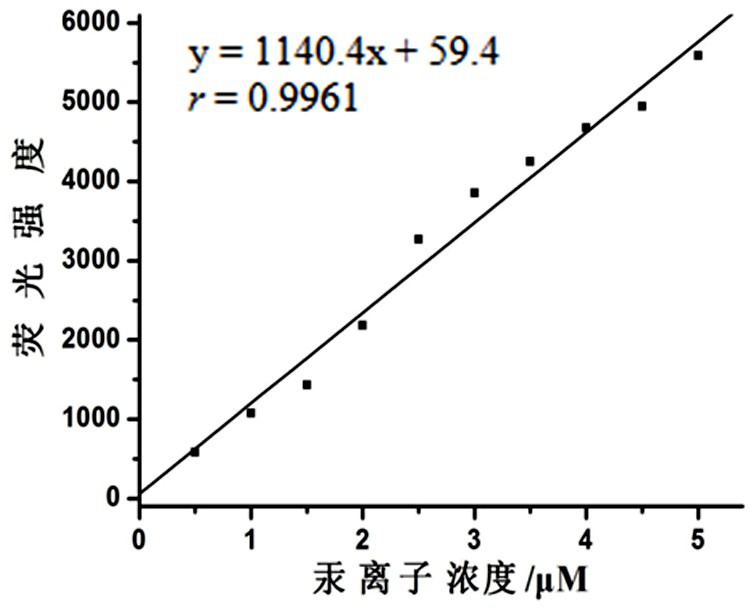
Image
Examples
Embodiment 1
[0034] Weigh 239.5 mg of rhodamine B (0.5 mmol), dissolve it in 11 mL of 1,2-dichloroethane, add 0.4 mL of phosphorus oxychloride dropwise, and heat to reflux at 95 °C for 4 h. After the reaction was completed, 1,2-dichloroethane was distilled off under reduced pressure to obtain a red rhodamine B acid chloride intermediate.
[0035] Weigh 136.0 mg N,N - Dimethylselenourea (0.9 mmol), which was dissolved in 6 mL of tetrahydrofuran, and 1.6 mL of triethylamine was added dropwise with stirring. The rhodamine B acid chloride prepared in the previous steps was dissolved in 5 mL of tetrahydrofuran, added dropwise to the above solution, and reacted at 20 °C for 8 h. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a purple-red substance, and then 10 mL of twice distilled water was added to it, extracted three times with dichloromethane (20 mL*3), and the solvent was distilled off under reduced pressure to obtain a crude product . Th...
Embodiment 2
[0043] Weigh 239.5 mg of Rhodamine B (0.5 mmol), dissolve it in 10 mL of 1,2-dichloroethane, add 0.3 mL of phosphorus oxychloride dropwise, and heat to reflux at 90 °C for 5 h. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a red rhodamine B acid chloride intermediate.
[0044] Weigh 75.5 mg N,N - Dimethylselenourea (0.5 mmol), which was dissolved in 5 mL of acetonitrile, and 1.2 mL of triethylamine was added dropwise with stirring. The rhodamine B acid chloride prepared in the previous steps was dissolved in 5 mL of tetrahydrofuran, added dropwise to the above solution, and reacted at 35 °C for 5 h. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a purple-red substance, and 10 mL of twice distilled water was added to it, and extracted three times with dichloromethane (20 mL*3), and the solvent was distilled off under reduced pressure to obtain a crude product. The obtained cru...
Embodiment 3
[0046] Weigh 239.5 mg of Rhodamine B (0.5 mmol), dissolve it in 12 mL of 1,2-dichloroethane, add 0.5 mL of phosphorus oxychloride dropwise, and heat to reflux at 100 °C for 5 h. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a red rhodamine B acid chloride intermediate.
[0047] Weigh 151.1 mg N,N - Dimethylselenourea (1.0 mmol), which was dissolved in 6 mL of acetonitrile, and 2.0 mL of triethylamine was added dropwise with stirring. The rhodamine B acid chloride prepared in the previous steps was dissolved in 6 mL of acetonitrile, added dropwise to the above solution, and reacted at 10 °C for 10 h. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a purple-red substance, and then 10 mL of twice distilled water was added to it, extracted three times with dichloromethane (20 mL*3), and the solvent was distilled off under reduced pressure to obtain a crude product . The obtained ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com