Anti-aggregation humanized IgG (immunoglobulin G) antibody CH2 structural domain mutant and application
An anti-aggregation and structural domain technology, applied in the direction of antibody mimics/scaffolds, anti-animal/human immunoglobulins, hybrid peptides, etc., can solve the problems of reduced drug efficacy, weak anti-aggregation ability, and poor stability, and achieve Good stability, good anti-aggregation ability, and the effect of reducing the risk of clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Amino acid sequence and nucleotide sequence of m01sm1
[0019] The m01sm1 skeleton provided by the present invention is obtained by the following method: firstly, a phage display library for random mutation of amino acids in the aggregation-prone region of m01s is constructed. Then, the phage display library was screened with specific anti-CH2 antibody to obtain candidate clones. Then it was sent for sequencing, and m01sm1 was obtained after identification.
[0020] The amino acid sequence of m01sm1 is Seq ID No.1, and the coding gene sequence is Seq ID No.2.
Embodiment 2
[0021] Example 2: Molecular conformation and existence form (monomer, dimer, etc.) of m01sm1
[0022] AKTA analysis of the existing form of m01sm1: Concentrate the purified m01sm1 protein to 1mg / ml, use PBS (pH7.4) as the elution buffer, pass through Column Superdex75Increase 10 / 300GL, and the flow rate is 1ml / min, and then determine the existing form testing, the result is figure 1 , compared with the standard curve, it can be found that the molecular weight of the protein is about 14kDa, and the result shows that they exist in the form of monomers.
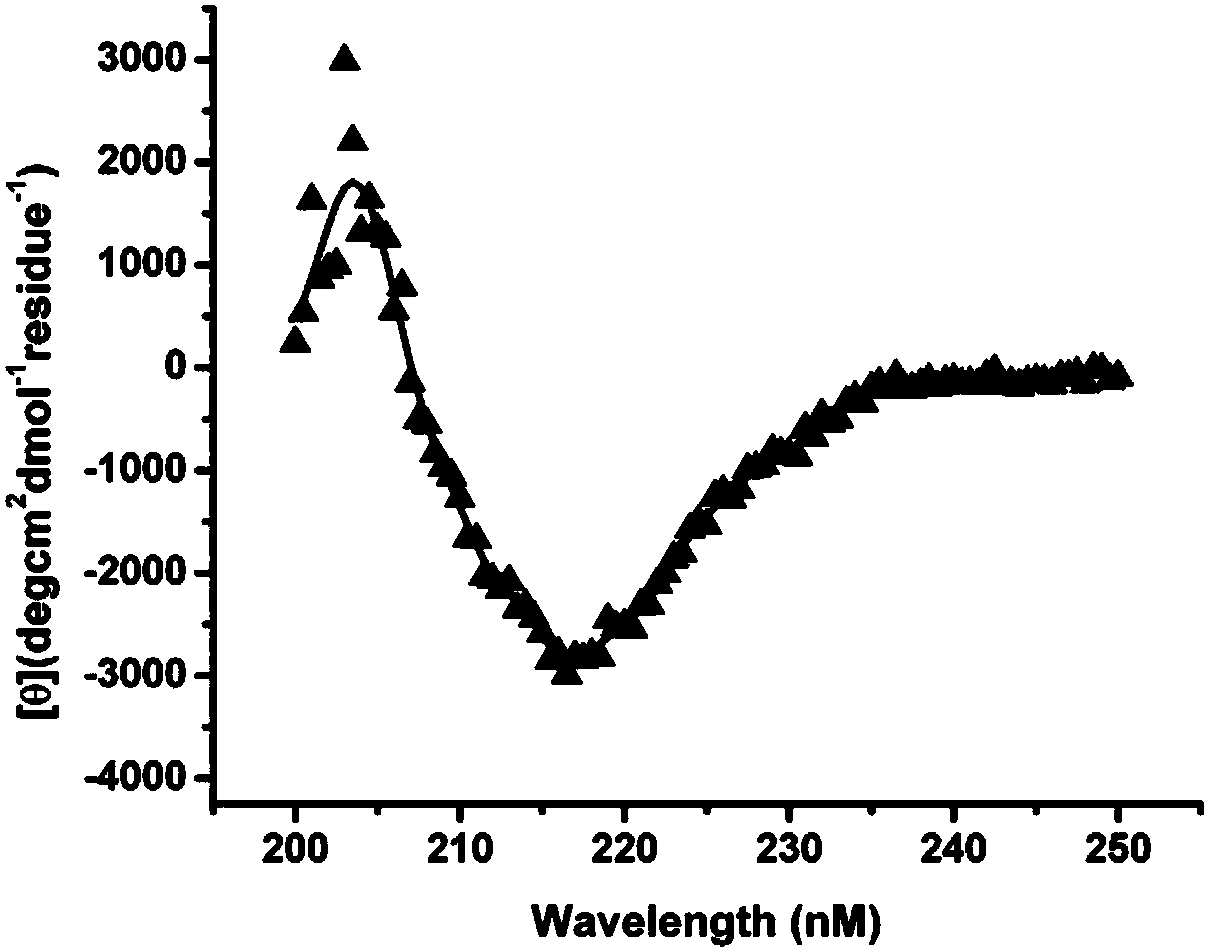
[0023] CD detection of protein molecular conformation: Dilute the m01sm1 protein to 0.3 mg / ml, PBS (pH7.4) as a control, and detect its average molar ellipticity under different wavelength conditions at the near ultraviolet end (wavelength λ=190nm~260nm), such as figure 2 , there is an obvious trough at λ=218nm, indicating that the secondary structure of the above protein is β-sheet.
Embodiment 3
[0024] Example 3: Comparison of m01s and m01sm1 anti-aggregation
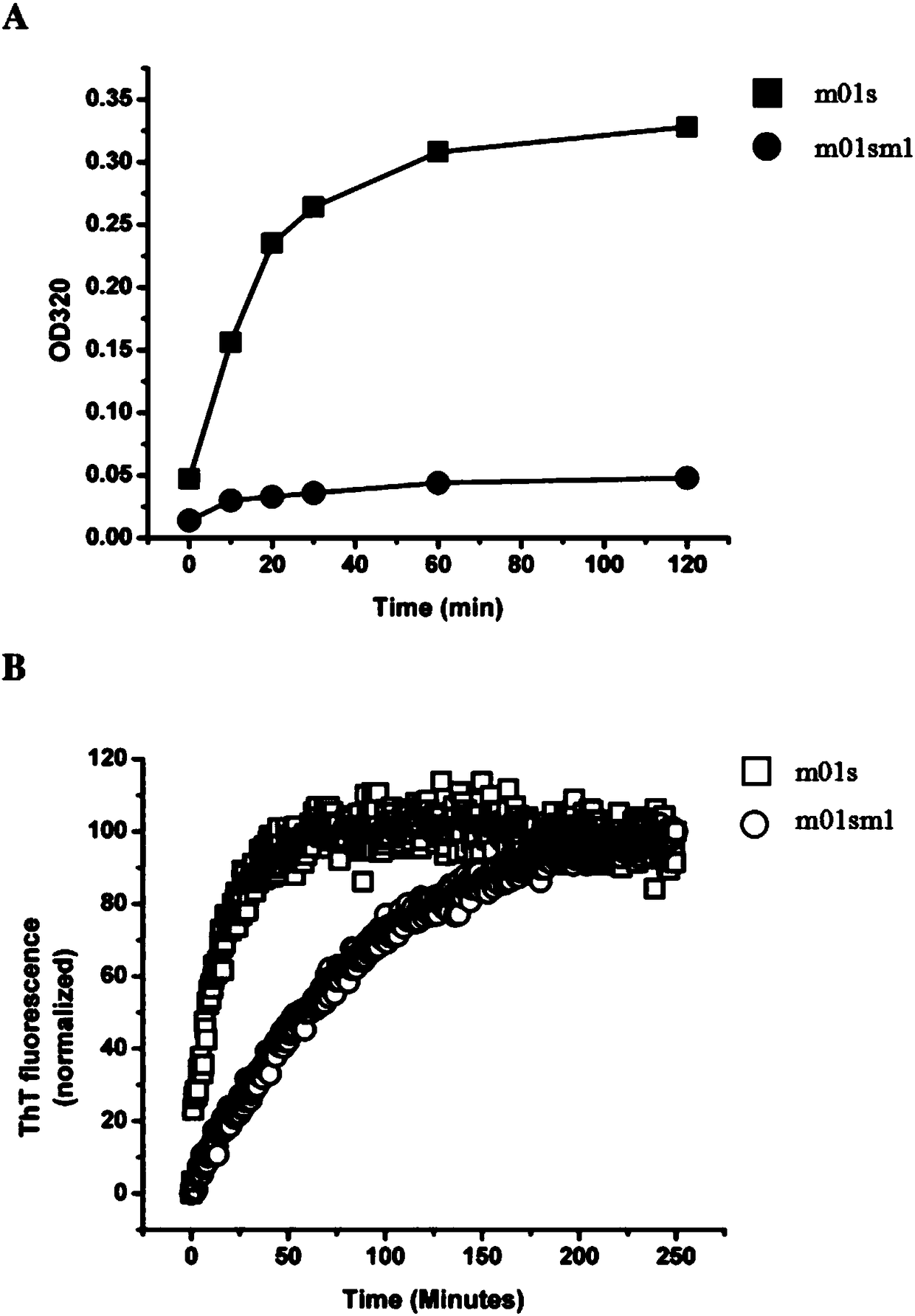
[0025] Turbidity experiment: Adjust the final protein concentration of m01s and m01sm1 to 1mg / ml, treat them in a water bath at 50°C, take samples at regular intervals, detect the absorbance at λ=320nm and record the data, among them, the PBS solution is used as a blank control, and the comparison The strength of anti-aggregation with m01s protein, from image 3 In A, it can be found that the m01sm1 signal rises slowly, indicating that its anti-aggregation ability is better.
[0026] ThT fluorescence experiment: adjust the final concentration of m01s and m01sm1 protein to 1mg / ml, add ThT, use 440nm as excitation light and 482nm as absorption light, and measure the fluorescence change within 250min. By measuring the luminescence intensity of m01sm1 and m01s proteins combined with ThT at different times, the anti-aggregation properties of m01sm1 and m01s proteins were compared, from image 3 In B, it can be fou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com