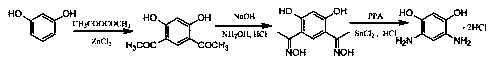
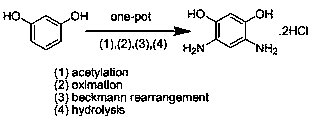
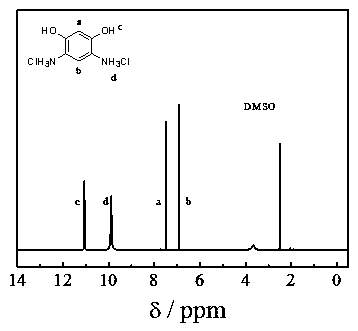
A kind of method for preparing 4,6-diaminoresorcinol hydrochloride by resorcinol one-pot method
A technology for diaminoresorcinol hydrochloride and resorcinol, which is applied in the field of preparing 4,6-diaminoresorcinol hydrochloride, can solve the problems of numerous steps, 4,6-diaminom-phenylene Diphenol hydrochloride is difficult to prepare and other problems, to achieve the effect of simple steps, avoiding the generation of nitro-substituted compounds, and simplifying the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] One-pot preparation of 4,6-diaminom-benzene at 100°C and the molar ratio of phosphorus pentoxide / methanesulfonic acid / hydroxylamine hydrochloride / acetic acid / resorcinol at 0 / 4 / 2 / 2 / 1 Diphenol hydrochloride.
[0025] Weigh resorcinol (5.50g) and acetic acid (6.00g) into a 100 mL three-neck flask, add 13mL (0.2mol) methanesulfonic acid, stir to dissolve, raise the temperature to 100°C, and react for 2 hours. Then gradually add hydroxylamine hydrochloride (7.00g) to it, then continue to react for 4 hours, then add hydrochloric acid (6mol / L, 40 mL) to it, continue to stir at 100°C, react for 2 hours, cool down, crystals are precipitated, and suction filtered , hydrochloric acid washing, ethanol washing, and vacuum drying to obtain 4,6-diaminoresorcinol hydrochloride with a yield of 5.0%.
Embodiment 2
[0027] One-pot preparation of 4,6-diaminom-benzene at 100°C and the molar ratio of phosphorus pentoxide / methanesulfonic acid / hydroxylamine hydrochloride / acetic acid / resorcinol at 0.30 / 2 / 2 / 2 / 1 Diphenol hydrochloride.
[0028] Weigh resorcinol (5.50g), P 2 o 5 (2.15g), acetic acid (6.00g) in a 100 mL three-necked flask, and add 6.5mL (0.1mol) methanesulfonic acid, stir to dissolve, raise the temperature to 120°C, and react for 2 hours. Then gradually add hydroxylamine hydrochloride (7.00g) to it, then continue to react for 4 hours, then add hydrochloric acid (6mol / L, 40 mL) to it, continue to stir at 100°C, react for 2 hours, cool down, crystals are precipitated, and suction filtered , hydrochloric acid washing, ethanol washing, and vacuum drying to obtain 4,6-diaminoresorcinol hydrochloride with a yield of 35%.
Embodiment 3~21
[0030] The difference between embodiment 3~21 and embodiment 1 mainly lies in the molar ratio difference of phosphorus pentoxide, methanesulfonic acid, hydroxylamine hydrochloride, acetic acid, resorcinol (resorcinol is 5.5g), and step (1 ), the reaction time and reaction temperature are different, and other conditions are the same as in Example 1, see Table 1 for details, wherein the acylating reagent used in Example 20 is acetic anhydride.
[0031] Table 1 Molar ratios, reaction conditions and yields of each component in Examples 1 to 21
[0032] Example temperature / ℃ P 2 o 5
Methanesulfonic acid Acylation reagent Resorcinol Hydroxylamine: an acylating reagent time / h Yield / % 1 100 0 4 2 1 1:1 2 5 2 100 0.30 2 2 1 1:1 2 35 3 100 0.70 4 3 1 1.5:1 3 65 4 100 1.50 8 5 1 2:1 5 68 5 120 0.30 2 3 1 1.5:1 5 47 6 120 0.70 4 5 1 2:1 2 92.5 7 120 1.50 8 2 1 1:1 3 90 8 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com