A kind of azelnidipine composition
A technology of azedipine and its composition, which is applied in the field of azedipine composition and its preparation, can solve the problems affecting tablet hardness, inhibition of the respiratory center, and potential safety hazards of patients, so as to solve the problem that the dissolution rate does not meet the standard and ensure the dissolution Performance, guaranteed stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
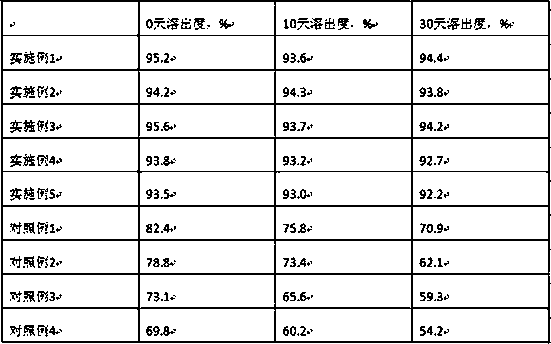
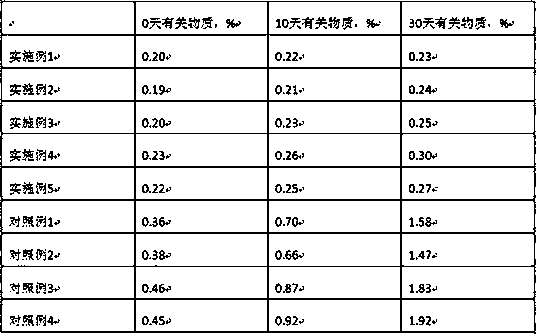
Examples
Embodiment 1
[0023] Embodiment 1. Azedipine 8g, mannitol 80g, low-substituted hydroxypropyl cellulose 30g, meglumine 5g, micropowder silica gel 30g, polysorbate 80 20g, magnesium stearate 1.6g, prepared according to the technical scheme part Methods 1000 tablets were prepared.
Embodiment 2
[0024] Embodiment 2. Azedipine 8g, mannitol 80g, low-substituted hydroxypropyl cellulose 30g, meglumine 4g, micropowder silica gel 30g, polysorbate 80 20g, magnesium stearate 1.6g, prepared according to the technical scheme part Methods 1000 tablets were prepared.
Embodiment 3
[0025] Embodiment 3. Azedipine 8g, mannitol 90g, low-substituted hydroxypropyl cellulose 26g, meglumine 5g, micropowder silica gel 30g, polysorbate 80 20g, magnesium stearate 1.6g, prepared according to the technical scheme part Methods 1000 tablets were prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com