Prucalopride succinate capsule and preparation method thereof
A technology of prucalopride and capsules, which is applied in the field of prucalopride succinate capsules and its preparation, achieving the effects of rapid disintegration, fewer types of excipients, and guaranteed drug stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
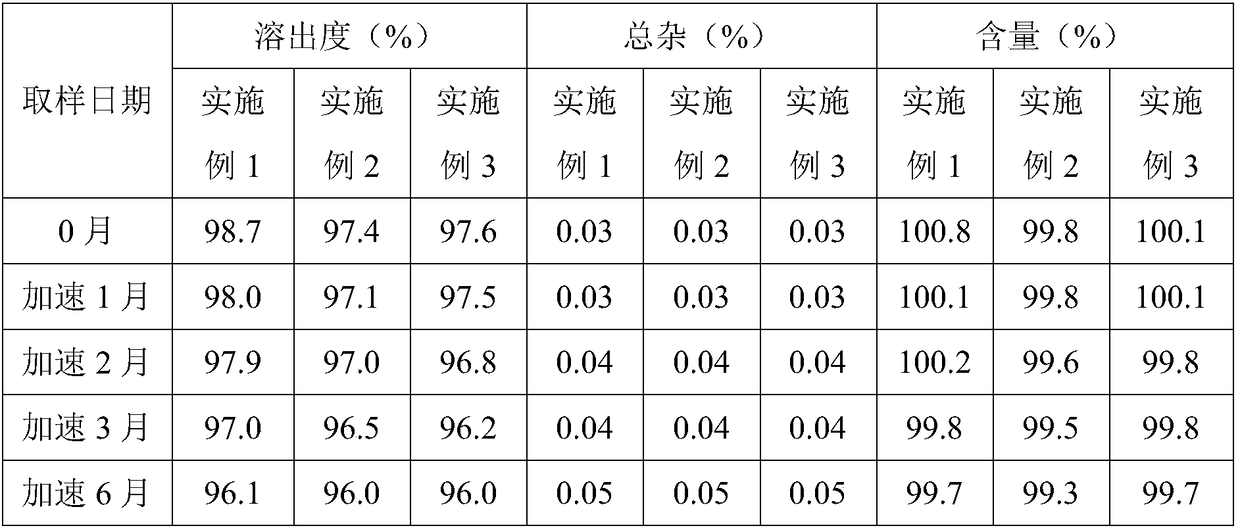
Embodiment 1
[0024] Embodiment 1 prucalopride succinate capsules and preparation method thereof
[0025] See Table 1 for the prescription of prucalopride succinate capsules.
[0026] Table 1
[0027] Element
weight percentage
prucalopride succinate
1%
100mg
lactose monohydrate
25%
2500mg
50%
5000mg
15%
1500mg
6%
600mg
3%
300mg
[0028] Preparation:
[0029] ① Pretreatment of raw and auxiliary materials: the raw materials are micronized, and the auxiliary materials are passed through an 80-mesh sieve for use;
[0030] ②Premix: Weigh prucalopride succinate, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and colloidal silicon dioxide according to the prescription quantity, and mix to obtain a uniform premix;
[0031] ③Total blending: Add magnesium stearate to the above prem...
Embodiment 2
[0034] Embodiment 2 prucalopride succinate capsules and preparation method thereof
[0035] See Table 2 for the prescription of prucalopride succinate capsules.
[0036] Table 2
[0037] Element
weight percentage
prucalopride succinate
1%
100mg
lactose monohydrate
35%
3500mg
Microcrystalline Cellulose PH101
40%
4000mg
15%
1500mg
6%
600mg
3%
300mg
[0038] Preparation:
[0039] ① Pretreatment of raw and auxiliary materials: the raw materials are micronized, and the auxiliary materials are passed through an 80-mesh sieve for use;
[0040] ②Premix: Weigh prucalopride succinate, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and colloidal silicon dioxide according to the prescription quantity, and mix to obtain a uniform premix;
[0041] ③Total blending: Add magnesium stearate to the abov...
Embodiment 3
[0044] Embodiment 3 prucalopride succinate capsules and preparation method thereof
[0045] See Table 3 for the prescription of prucalopride succinate capsules.
[0046] table 3
[0047] Element
weight percentage
prucalopride succinate
1%
100mg
20%
2000mg
55%
5500mg
15%
1500mg
6%
600mg
Magnesium stearate
3%
300mg
[0048] Preparation:
[0049] ① Pretreatment of raw and auxiliary materials: the raw materials are micronized, and the auxiliary materials are passed through an 80-mesh sieve for use;
[0050] ②Premix: Weigh prucalopride succinate, lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and colloidal silicon dioxide according to the prescription quantity, and mix to obtain a uniform premix;
[0051] ③Total blending: Add magnesium stearate to the above prem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com