Hindered amine antioxidant preparation method
A technology of hindered amines and antioxidants, applied in the field of organic chemical synthesis, can solve the problems of harsh preparation conditions of antioxidants, poor compatibility of antioxidants, easy dissolution, etc., and achieve good anti-solvent extraction performance and not easy to color , good compatibility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
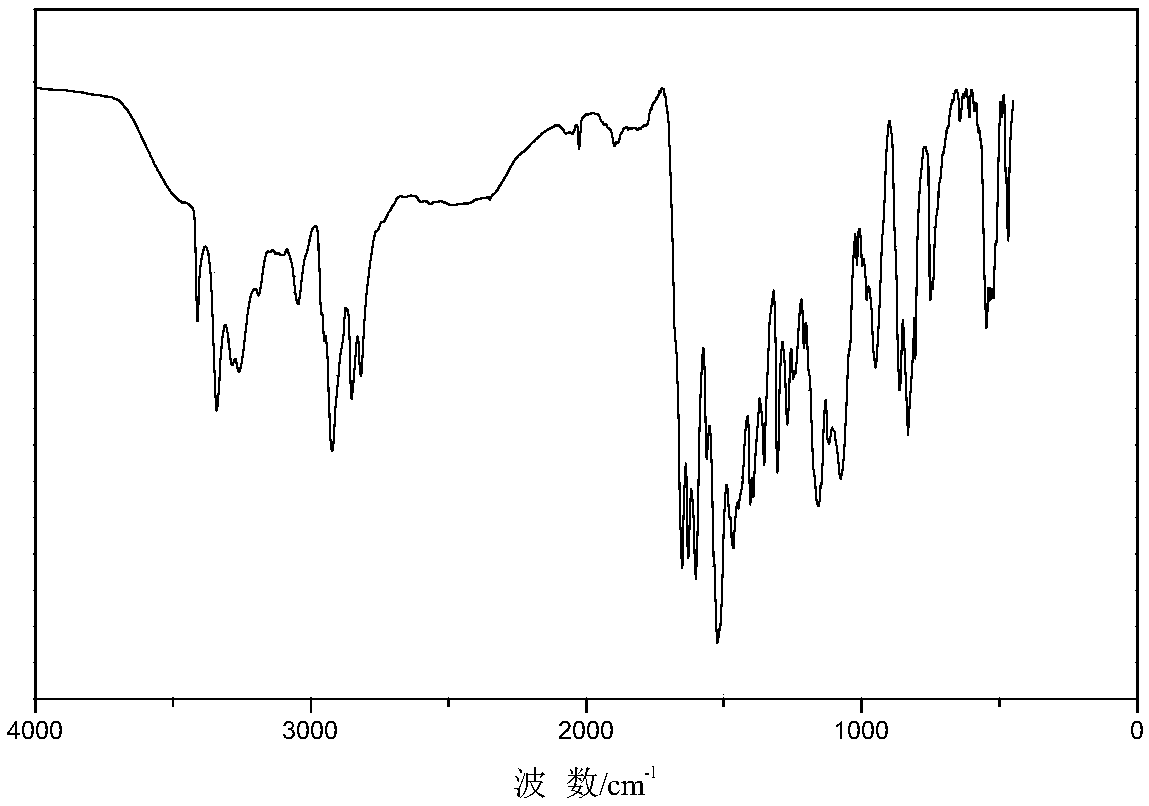
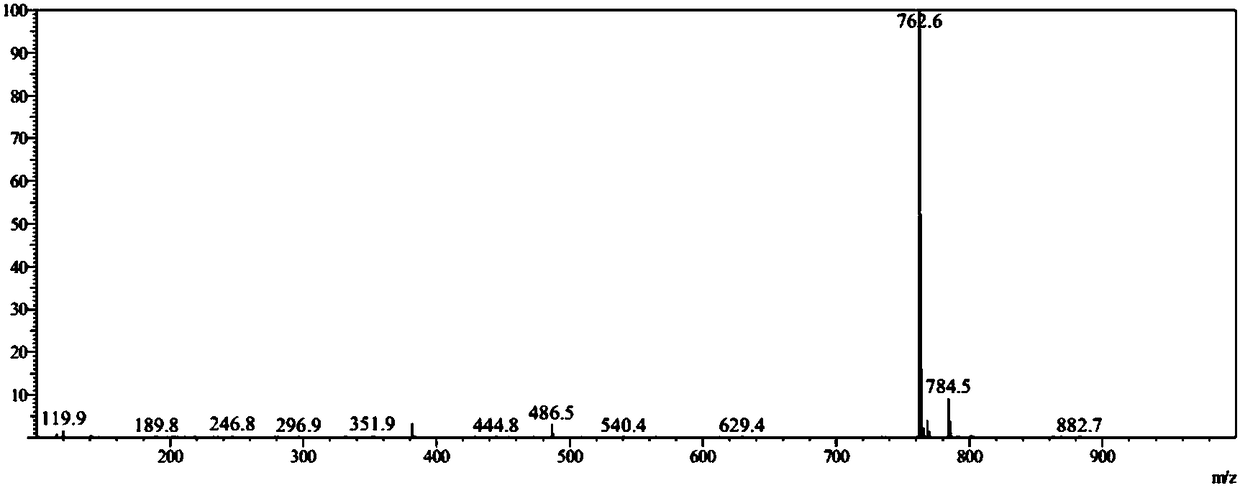
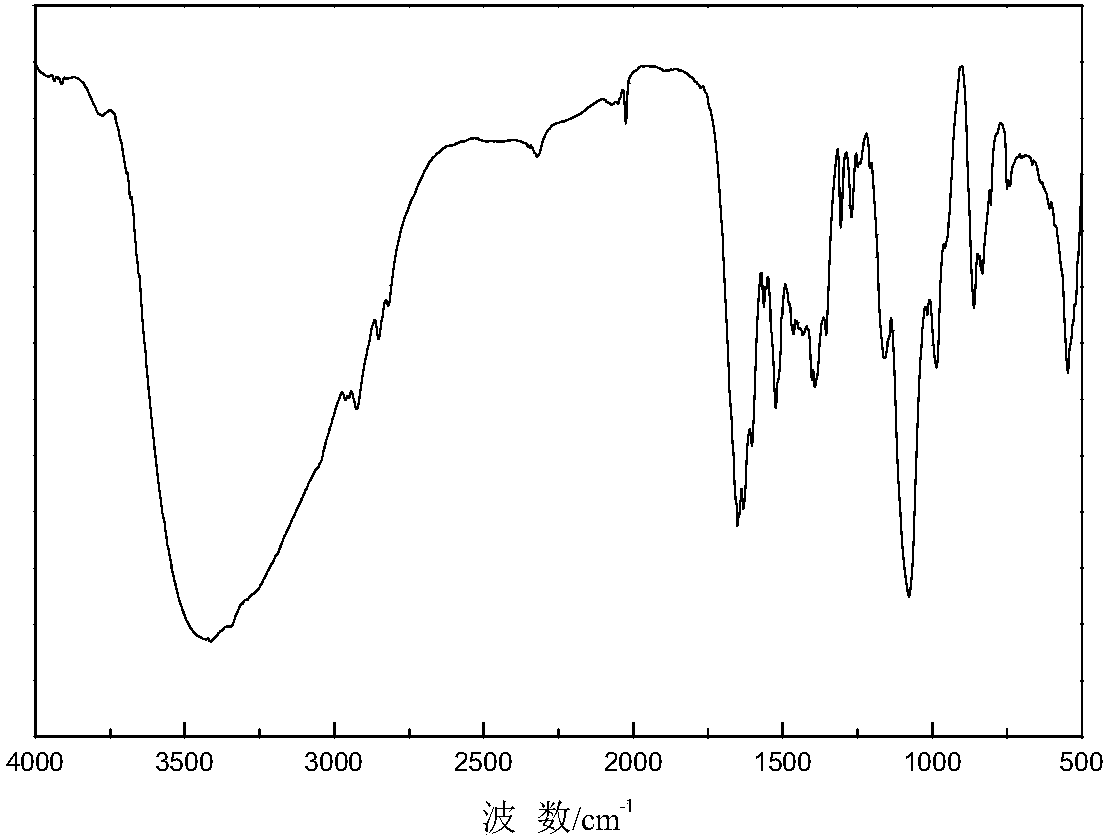
Image
Examples
preparation example Construction
[0036] The invention discloses a method for preparing hindered amine antioxidants, and the method specifically includes:
[0037] (1) Synthesis of intermediate (Ⅰ): First, dissolve the p-haloaniline compound and the accelerator N,N-diisopropylethylamine in tetrahydrofuran and drop them at low temperature (preferably -5℃~5℃) Add acid halide compound, react at room temperature for a certain period of time (preferably 3-7h), after the completion of the reaction, add a certain amount of saturated NH to the reaction mixture 4 Cl aqueous solution was used to stop the reaction, and then the organic phase was extracted with a certain amount of ethyl acetate. 2 SO 4 Drying, and then drying under vacuum conditions, to obtain intermediate (I).
[0038] (2) Synthesis of intermediate (Ⅱ): Dissolve intermediate (Ⅰ) in ethanol, N 2 Protect, add dropwise the ethanol solution of chain fatty amine at room temperature, react at 70-90°C for 60-80h, the reaction mixture is concentrated under vacuum, and...
Embodiment 1
[0045] This embodiment provides a method for preparing a novel hindered amine antioxidant, which includes the following steps:
[0046] Step 1, accurately weigh 60g of p-bromoaniline and 67.6g of N,N-diisopropylethylamine and dissolve in 1.5L of tetrahydrofuran, slowly add 34.7g of acryloyl chloride dropwise while stirring at 0°C, and react for 4h at 25°C. Add saturated NH to the reaction mixture 4 1L Cl solution was used to stop the reaction, and then extracted with 3L ethyl acetate, Na 2 SO 4 After dehydration, filtration, and vacuum drying at 30°C, N-(4-bromophenyl)acrylamide (I) is obtained.
[0047] Step two, accurately weigh out 60g of N-(4-bromophenyl)acryloyl chloride and dissolve it in 1.0L of ethanol. 2 Under protection, 24.6g of dodecylamine was slowly added dropwise while stirring at 25°C, and reacted for 72h at 85°C. The reaction mixture was concentrated in vacuo and purified by silica gel column chromatography. The eluent used was petroleum ether: ethyl acetate = 10:1...
Embodiment 2
[0056] Step one: accurately weigh 60g of p-bromoaniline and 67.6g of N,N-diisopropylethylamine, dissolve in 1.5L of tetrahydrofuran, slowly add 39.5g of acryloyl chloride dropwise while stirring at 0°C, and react for 4h at 25°C. Add saturated NH to the reaction mixture 4 1L Cl solution was used to stop the reaction, and then extracted with 3L ethyl acetate, Na 2 SO 4 After dehydration, filtration, and vacuum drying at 30°C, N-(4-bromophenyl)acrylamide (I) is obtained.
[0057] Step two, accurately weigh out 60g of N-(4-bromophenyl)acryloyl chloride and dissolve it in 1.0L of ethanol. 2 Under protection, 26.5g n-tetradecylamine was slowly added dropwise while stirring at 25°C, and reacted for 75h at 85°C. The reaction mixture was concentrated in vacuo and purified by silica gel column chromatography. The eluent used was petroleum ether: ethyl acetate = 10:1 to obtain 3,3'-tetradecylamine-bis-(4-bromophenylhydrazine) ) Propionamide (II).
[0058] Step 3: Weigh accurately 28.2g 3,3'-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com