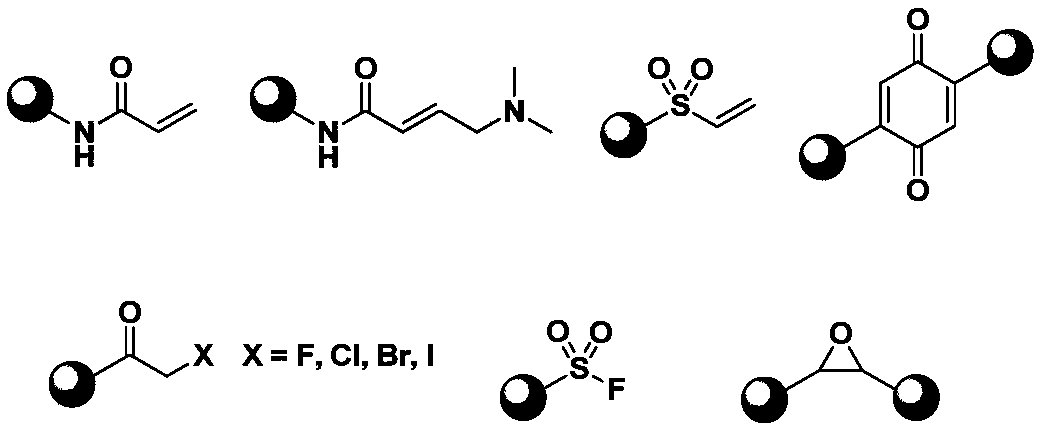
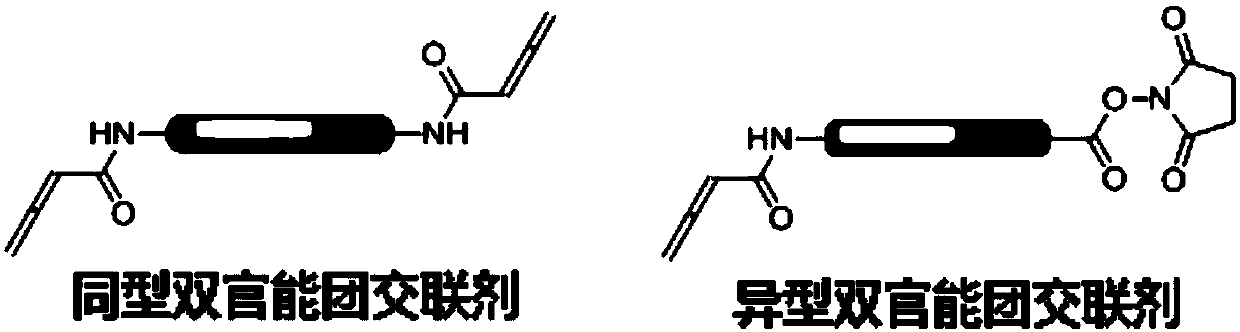
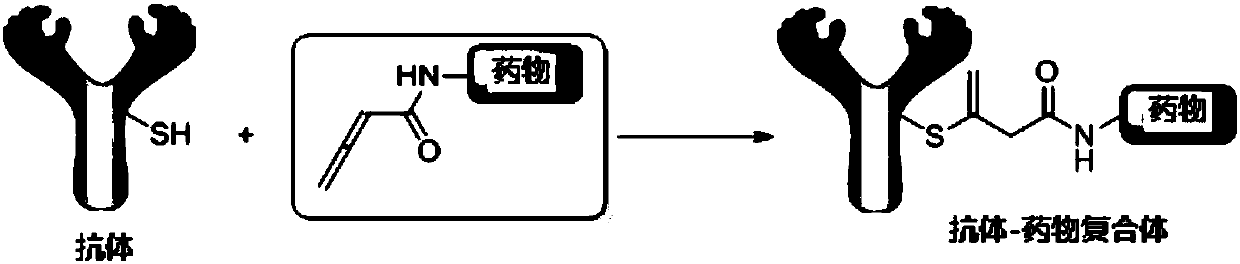
Application of compound containing allenamide group in preparation of protein inhibitors, protein cross-linkers or protein markers
A technology of allenamide group and allenamide, which is applied in the field of biochemistry, can solve the problems of slow reaction rate between acrylamide and cysteine, affecting selectivity, etc., and achieves strong application value, simple addition product and stable structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of EGFR covalent inhibitor ACI-1
[0049]
[0050] 3-Butynoic acid (168 mg, 2 mmol) was dissolved in DCM (2.0 mL), and DMF (cat., 16 μL) and oxalyl chloride (203 μL, 2.4 mmol) were gradually added dropwise to the solution at 0°C. The reaction was warmed to room temperature and stirred at room temperature for 2 hours. After the reaction, the solvent and excess oxalyl chloride were removed under reduced pressure, and the resulting residue was redissolved in DCM (4 mL). This solution was then added dropwise to a solution of compound 1-1 (254 mg, 0.8 mmol) and triethylamine (0.84 mL, 6 mmol) in THF / DCM (50 mL / 6 mL) at 0° C. for about 20 minutes. After the reaction was stirred at 0°C for 1 h, the reaction was washed with saturated NaHCO 3 The solution (15 mL) was quenched and the organic phase was collected. The aqueous phase was further extracted with ethyl acetate (30 mL×3). The organic phases were combined and washed with saturated brine (20 mL...
Embodiment 2
[0055] The computer simulation of the combination of EGFR and ACI-1 obtained in Example 1 shows that the distance between the enamide in ACI-1 and the 797-position cysteine in the EGFR binding pocket is relatively close, which provides a basis for the formation of covalent bonds.
[0056] In order to prove that they can indeed form covalent linkages, the EGFR kinase protein was further co-incubated with ACI-1 obtained in Example 1 for 6 hours at 37°C, and at the same time DMSO was used to replace ACI-1 as a negative control, and then the samples were Perform liquid phase tandem mass spectrometry analysis, the results are as follows Image 6 shown. The results showed that the molecular weight of the chymotrypsin hydrolyzed peptide containing cysteine 797, the experimental group (ACI-1 treatment) increased the molecular weight of ACI-1 compared with the control group (DMSO treatment). This result proves that ACI-1 can form a covalent binding with EFGR, ACI-1 is a covalent E...
Embodiment 3
[0059] In order to prove the antitumor effect of its EGFR covalent inhibitor ACI-1, the present invention is tested on lung cancer cell line (NCI-H1975), and the results are as follows: Figure 7 shown. Further tests were carried out on the nude mouse model, and the results were as follows Figure 8 shown.
[0060] The experimental results show that ACI-1 has excellent anti-tumor effect, and the effect is significantly better than the existing anti-tumor drug Gefitinib.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com