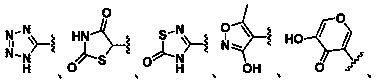
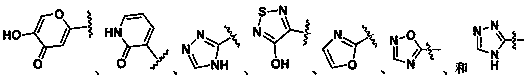
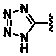
Inhibitors of indoleamine 2,3-dioxygenase for the treatment of cancer
A C3-C8, C2-C10 technology, applied in the field of indoleamine 2,3-dioxygenase inhibitors for the treatment of cancer, can solve problems such as increased tumor microenvironment tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0268] The preparation of prodrugs is well known in the art and described, for example, in King, F.D., ed., Medicinal Chemistry: Principles and Practice, The Royal Society of Chemistry, Cambridge, UK (2nd ed., reprinted 2006); Testa, B. et al., Hydrolysis in Drug and Prodrug Metabolism. Chemistry, Biochemistry and Enzymology, VCHA and Wiley-VCH, Zurich, Switzerland (2003); Wermuth, C.G., ed., The Practice of Medicinal Chemistry, 3rd Edition, Academic Press, San Diego , CA (2008).
[0269] The present invention is intended to include all isotopes of atoms present in the compounds of the present invention. Isotopes include those atoms having the same atomic number but different mass numbers. By way of general example and not limitation, isotopes of hydrogen include deuterium and tritium. Isotopes of carbon include 13 C and 14c. Isotopically-labeled compounds of the invention can generally be prepared using an appropriate isotopically-labeled reagent in place of an otherwise...
Embodiment 1
[0515] 3-(3-((4-chlorophenyl)amino)-4-(ethyl(4-hydroxy-4-methylcyclohexyl)amino)phenyl)-3-methylbutanoic acid
[0516]
[0517] 1A.8-Methyl-1,4-dioxaspiro[4.5]decane-8-ol
[0518] Cool a stirred solution of 1,4-dioxaspiro[4.5]decane-8-one (5 g, 32.0 mmol) in dry THF (70 mL) to -70 °C and add methyl bromide in ether dropwise over 10 min Magnesium (23.48 mL, 70.4 mmol). The cooling bath was allowed to warm to room temperature and the mixture was stirred overnight. use NH 4 The mixture was quenched with saturated aqueous Cl (75 mL) and extracted with ether (2 x 300 mL). The combined ether extracts were washed with brine (50 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 1A (yellow liquid, 5.1 g, 29.6 mmol, 92% yield), which was used in the next step without Further purification. 1 H NMR (400MHZ, CDCl 3 )δ3.96-3.90(m, 4H), 1.91-1.84(m, 2H), 1.71-1.65(m, 3H), 1.63-1.57(m, 3H), 1.22(s, 3H).
[0519] 1B. 4-Hydroxy-4-methylcyclohex...
Embodiment 2-4
[0541]
[0542] Examples 2-4 were prepared from 1H diastereomer 2 and the corresponding halide following the procedure described in Synthetic Example 1 .
[0543]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com