Indolocarbazole alkaloids as well as preparation method and application thereof
A technology of indolecarbazole and alkaloids, which is applied in the field of preparation of active compounds from secondary metabolites of actinomycetes, can solve problems such as poor selectivity, and achieve the effects of simple operation, easy cultivation, and easy expansion of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1. the fermentation of actinomycetes
[0035] The actinomycete adopts the streptomyces Streptomycessp.CICC 11031 sold by China Common Microorganism Culture Collection and Management Center;
[0036] 1) Inoculate the actinomycetes in Gao's No. 1 medium, culture at 28°C, and culture on a shaker at 180rpm for 4-6 days to obtain seed liquid;
[0037] 2) Inoculate the seed solution obtained in step 1) into rice culture medium (rice culture medium, made of the following components: 40 g of rice mass; 60 mL of seawater salt water, obtained by high-pressure damp heat sterilization after being placed in a 500 mL Erlenmeyer flask), The inoculum size of each bottle is 10 mL, and the culture is statically cultured at 28° C. for 70 days. The culture is soaked in ethyl acetate, extracted and concentrated to obtain a fermented crude extract containing the compound of the present invention.
Embodiment 2
[0038] Embodiment 2. The preparation of compound
[0039] The obtained fermented crude extract is subjected to gel column chromatography (the filler is hydroxypropyl sephadex LH-20), and the eluent is methanol- In the water system, each 1 / 4 column volume is a fraction, and the fractions containing the target compound are combined for TLC analysis. Medium-pressure preparative chromatographic separation (Sepax Amethyst C-18 (10μm, 30×400mm) chromatographic column, detection wavelength 291nm, filler is octadecylsilane bonded silica gel), mobile phase is 40%-100% by volume methanol+ Gradient elution with 0.05% TFA-water solution, TLC analysis The part containing staurosporine and the part not containing staurosporine were combined respectively, and the new compound was located in the part not containing staurosporine. The staurosporine-free fraction was subjected to silica gel column chromatography (300-400 mesh), and the eluent was a methanol-dichloromethane system (methanol:dic...
Embodiment 3
[0041] Example 3. Identification of Compounds
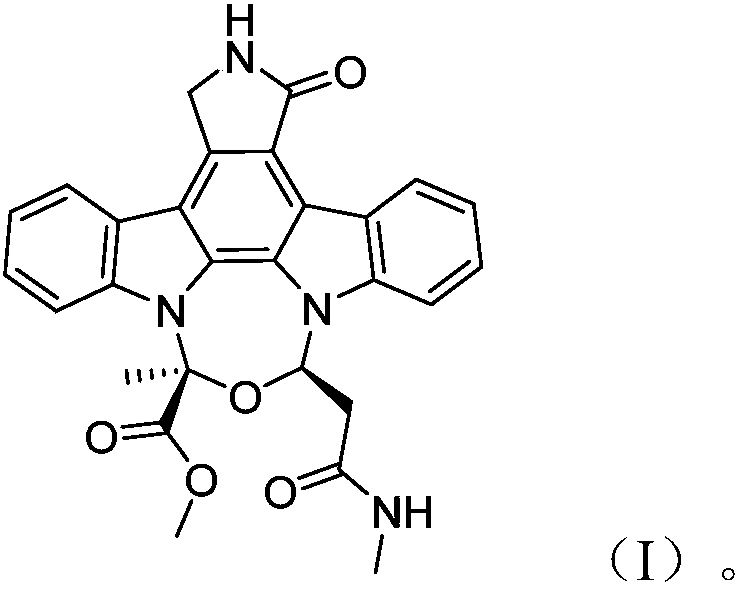
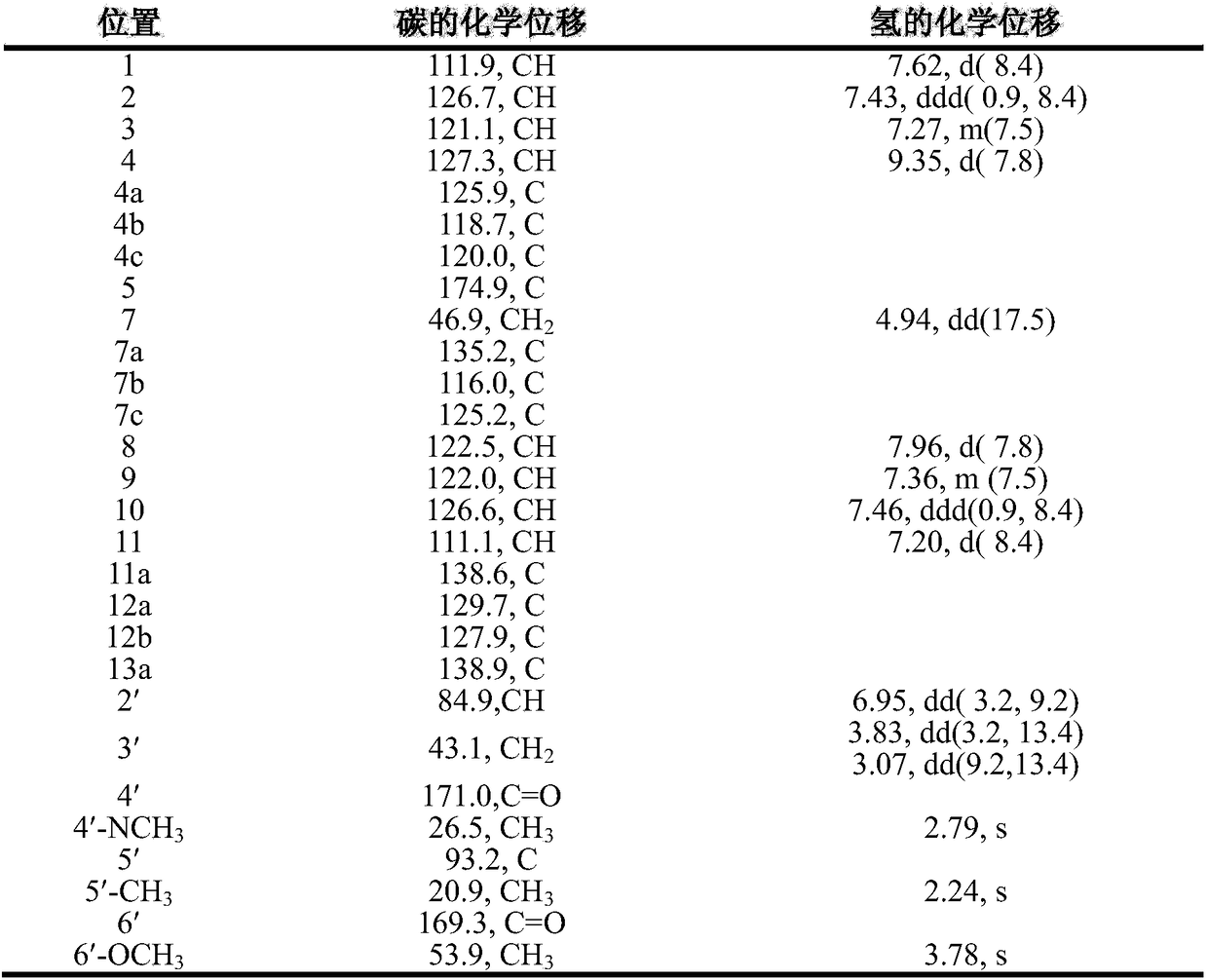
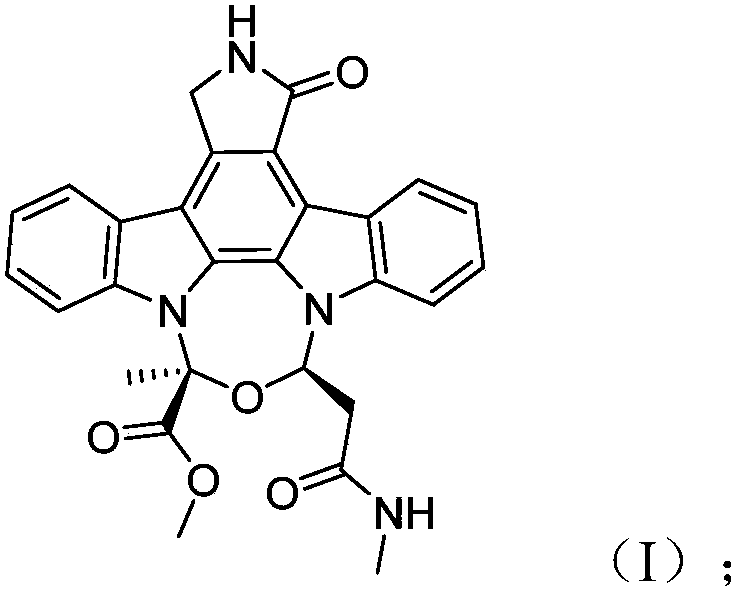
[0042] The compound of the present invention is light yellow powder, high-resolution mass spectrometry HR-ESI-MS gives quasi-molecular ion peak m / z497.1819[M+H] + (calculated 497.1825), suggesting that the molecular formula is C 28 h 24 N 4 o 5 , named actinocarbazole B, the specific structural analysis results are shown in Table 1 below:
[0043] Table 1
[0044]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com