Core-shell-structured catalyst for fuel cell and preparation and application of catalyst
A fuel cell, core-shell structure technology, applied in the field of electrochemistry, can solve problems such as limiting the commercialization of fuel cells, and achieve the effects of good corrosion resistance and anti-dissolution ability, low energy consumption, and dense coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Using the method of the present invention, using carbon-supported palladium nanoparticles as a substrate, a carbon-supported platinum-copper-palladium core-shell catalyst was prepared in a mixed electrolyte solution containing a soluble subplatinous salt.
[0030] 1) Microscopic morphology characterization of the prepared catalyst:
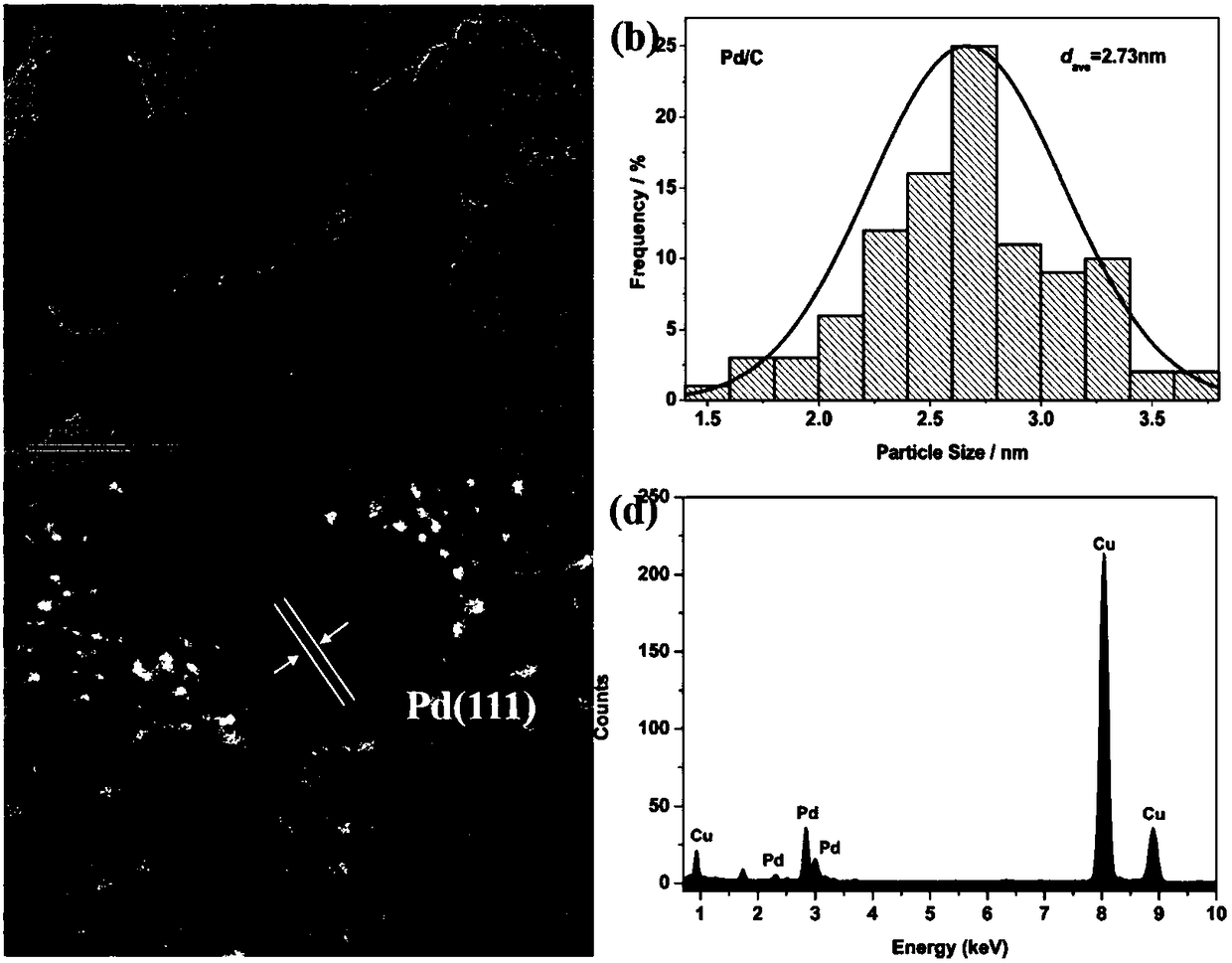
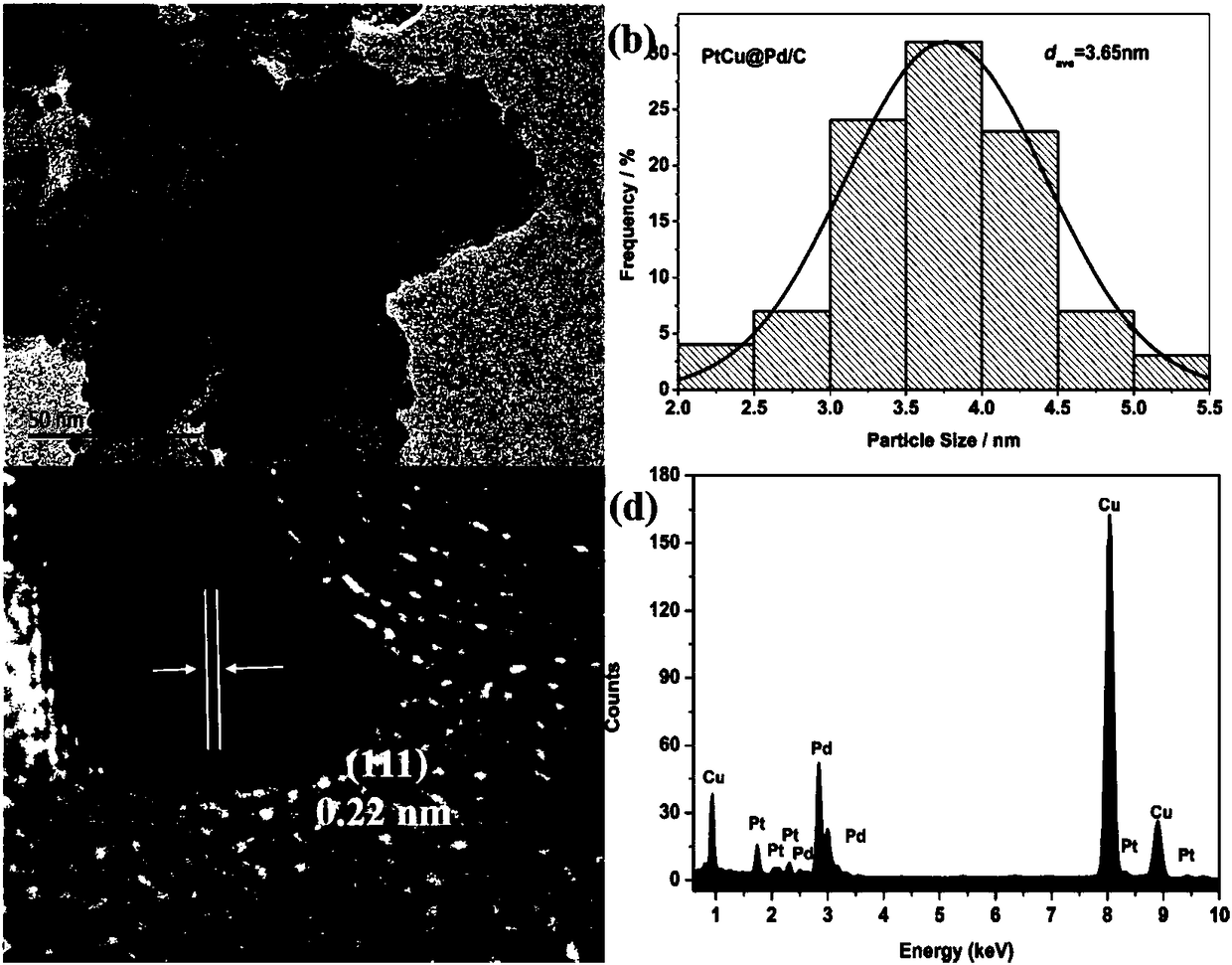
[0031] The copper-modified platinum shell-palladium core catalyst particles prepared by the method of the present invention are evenly distributed on the carbon carrier, with a uniform size and an average particle diameter of 3.65nm, as shown in the attached figure 2 shown. attached figure 1 Shown is the transmission electron microscope photo of carbon-supported palladium nanoparticles. The palladium nanoparticles are evenly distributed on the carbon support, with an average particle size of 2.73nm. Therefore, the thickness of the platinum shell is calculated to be 0.46nm, which is about 2 platinum atomic layers thick. The hig...
Embodiment 2
[0037] Example 2: Using the method described in the present invention, a catalyst with a core-shell structure is prepared in a mixed electrolyte solution containing a soluble platinum sub-salt, using a nanoporous gold skeleton as a substrate.
[0038] 1) Microscopic morphology characterization of the prepared catalyst:
[0039] as attached Figure 5 Shown, (a) figure and (b) figure are nanoporous gold and take nanoporous gold as substrate, utilize the surface shape of the core-shell catalyst of platinum-copper shell cladding gold framework that the method for the present invention makes respectively appearance. Nanoporous gold has a three-dimensional continuous through-pore structure with a clear skeleton and a pore size distribution in the range of 30-50nm. The microstructure of the nanoporous gold-loaded copper-modified platinum layer catalyst prepared by this method is similar to that of nanoporous gold, but the surface morphology has changed, and the structural integrity...
Embodiment 3
[0044] Embodiment 3: Using the method of the present invention, using the nanoporous gold framework as the substrate, first prepare the palladium shell layer in the mixed electrolyte solution containing the soluble sub-palladium salt, and then use this as the substrate to prepare the palladium shell layer in the mixed electrolyte solution containing the soluble sub-palladium salt. Preparation of core-shell catalysts with platinum shells in electrolyte solution.
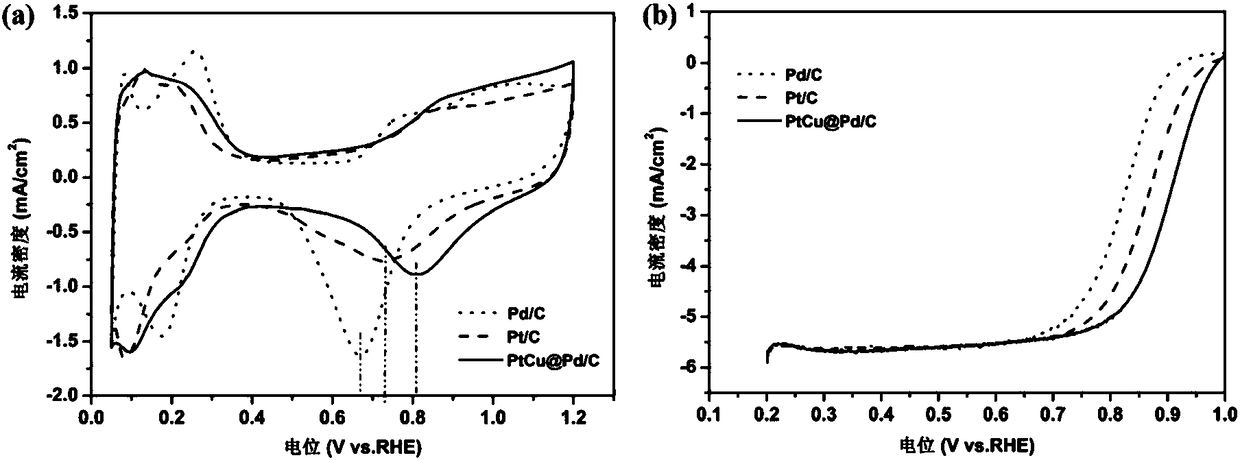
[0045] Utilize the CV characterization of the core-shell structure catalyst prepared by the method of the present invention:
[0046] First, a glassy carbon electrode loaded with nanoporous gold is used as a working electrode, and a core-shell structure catalyst with a palladium shell layer covering a gold skeleton is prepared in an electrolyte containing a soluble sub-palladium salt by using the preparation method of the present invention; Layer-coated gold skeleton is the substrate, transferred to the electrolyte co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Aperture size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com