Method for measuring dissolution rate of obeticholic acid tablets
A technology of obeticholic acid tablets and obeticholic acid, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of insufficient detection sensitivity, low content of dissolution samples of obeticholic acid tablets, etc., and achieve simple reaction conditions Ease of operation, high sensitivity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Methodology Validation of Dissolution Detection of Obeticholic Acid Tablets
[0048] 1 Selection of measurement wavelength
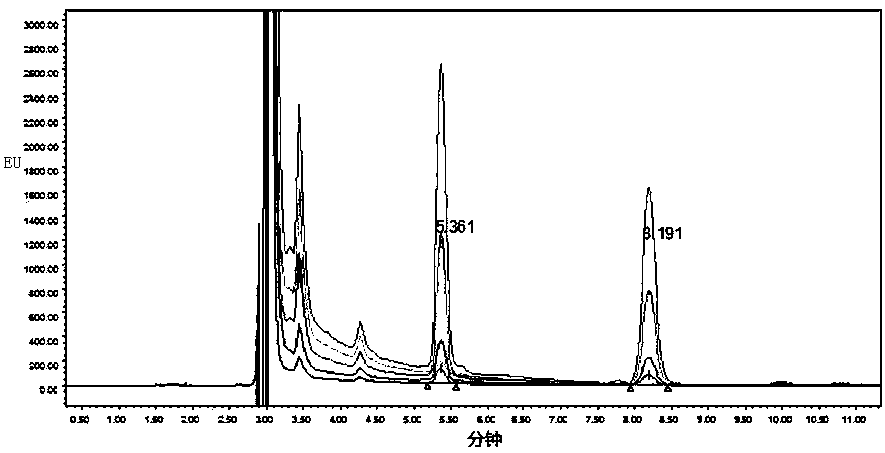
[0049] Instruments and reagents: Waters 2695 liquid chromatograph, Tianda Tianfa RCZ-8m dissolution tester, aglent phenyl chromatography column (4.6*250mm, 5μm); acetonitrile-methanol is chromatographically pure, water is ultrapure water, other reagents For analytical purity.
[0050] Prepare the test solution, use the excitation wavelength of 420nm to scan the absorption wavelength of 440 nm to get the maximum value, then use the obtained value to return to scan the excitation wavelength, and then use the newly obtained excitation wavelength to scan the absorption wavelength to obtain a suitable detection wavelength range: The excitation wavelength is 384nm~404nm, the emission wavelength is 421nm~440nm, and the most sensitive wavelength: the excitation wavelength is 384nm, and the emission wavelength is 421nm.
[0051] 2 Detection lim...
Embodiment 2
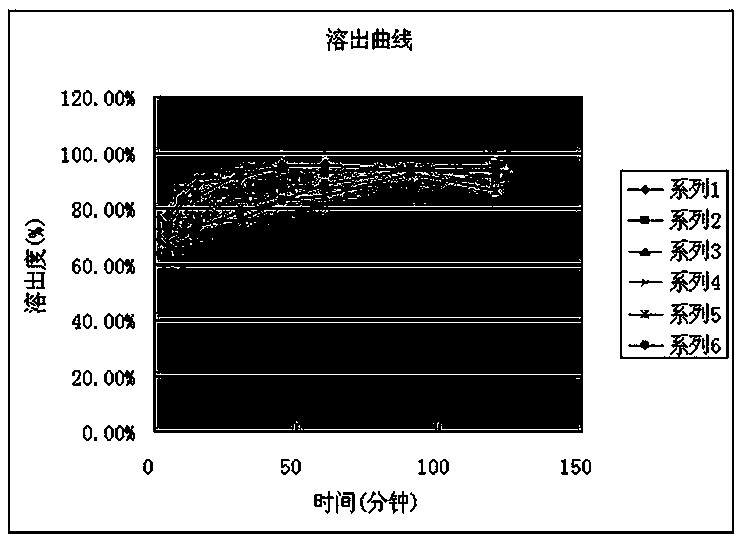
[0058] Example 2 Determination of the Dissolution Profile of Obeticholic Acid Tablets in Phosphate Buffered Saline
[0059] Step 1, solution preparation
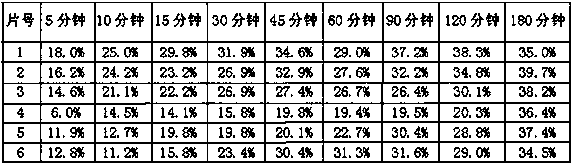
[0060] Preparation of the test solution: Take 6 units of obeticholic acid tablets (specification 5 μg), use the second method device of the dissolution method (Chinese Pharmacopoeia 2015 edition four), use pH 6.8 phosphate buffer 900ml as the dissolution medium , the rotating speed is 50 revolutions per minute, operate according to the law, take 5ml of the solution at 5 minutes, 10 minutes, 15 minutes, 30 minutes, 45 minutes, 60 minutes, 90 minutes, and 120 minutes, filter, and accurately measure the continued filtrate as the supply Test solution.
[0061] Preparation of the reference substance solution: Precisely measure the appropriate amount of the reference substance, add the corresponding dissolution medium to make solutions containing 1 μg, 2 μg, 5 μg, 7 μg, and 10 μg of obeticholic acid in each 1 ml respectively as a...
Embodiment 3
[0085] Example three: Determination of the dissolution rate of obeticholic acid tablets in water
[0086] 1 solution preparation
[0087] Preparation of the test solution: Take 6 units of obeticholic acid tablets, use the second method device of the dissolution method (Chinese Pharmacopoeia 2015 edition four), use 900ml water as the dissolution medium, and the speed is 50 rpm, and operate according to the law , get solution 5ml respectively in 5 minutes, 10 minutes, 15 minutes, 30 minutes, 45 minutes, 60 minutes, 90 minutes, 120 minutes, filter, and precision measures continued filtrate as need testing solution.
[0088] 2 Preparation of the reference substance solution: Accurately measure an appropriate amount of the reference substance, add the corresponding dissolution medium to make a solution containing about 3 micrograms of obeticholic acid per 1 ml as the reference substance solution.
[0089] 3 Extraction and evaporation: Accurately measure 5ml of the test solution an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com